 PDF(1517 KB)
PDF(1517 KB)


DNA分子“光开关”含钌多吡啶络合物细胞摄取、胞内分布及毒性机理研究
朱本占, 肖璇, 巢细娟, 唐苗, 黄蓉, 邵杰
化学进展 ›› 2018, Vol. 30 ›› Issue (12) : 1975-1991.
 PDF(1517 KB)
PDF(1517 KB)
 PDF(1517 KB)
PDF(1517 KB)
DNA分子“光开关”含钌多吡啶络合物细胞摄取、胞内分布及毒性机理研究
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Investigation of the Mechanism of Cellular Uptake, Distribution and Toxicity of the DNA ‘Light-Switch’ Ru(Ⅱ) Polypyridyl Complexes
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}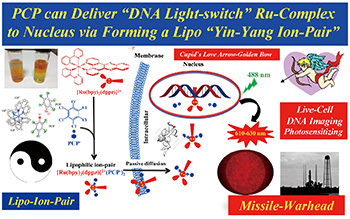
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |