 PDF(8957 KB)
PDF(8957 KB)


非富勒烯受体DA'D型稠环单元的结构修饰及电池性能研究
薛朝鲁门, 刘宛茹, 白图雅, 韩明梅, 莎仁, 詹传郎
化学进展 ›› 2022, Vol. 34 ›› Issue (2) : 447-459.
 PDF(8957 KB)
PDF(8957 KB)
 PDF(8957 KB)
PDF(8957 KB)
非富勒烯受体DA'D型稠环单元的结构修饰及电池性能研究
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Recent Progress on Solar Cell Performance Based on Structural Tailoring on DA'D Units of Nonfullerene Acceptors
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}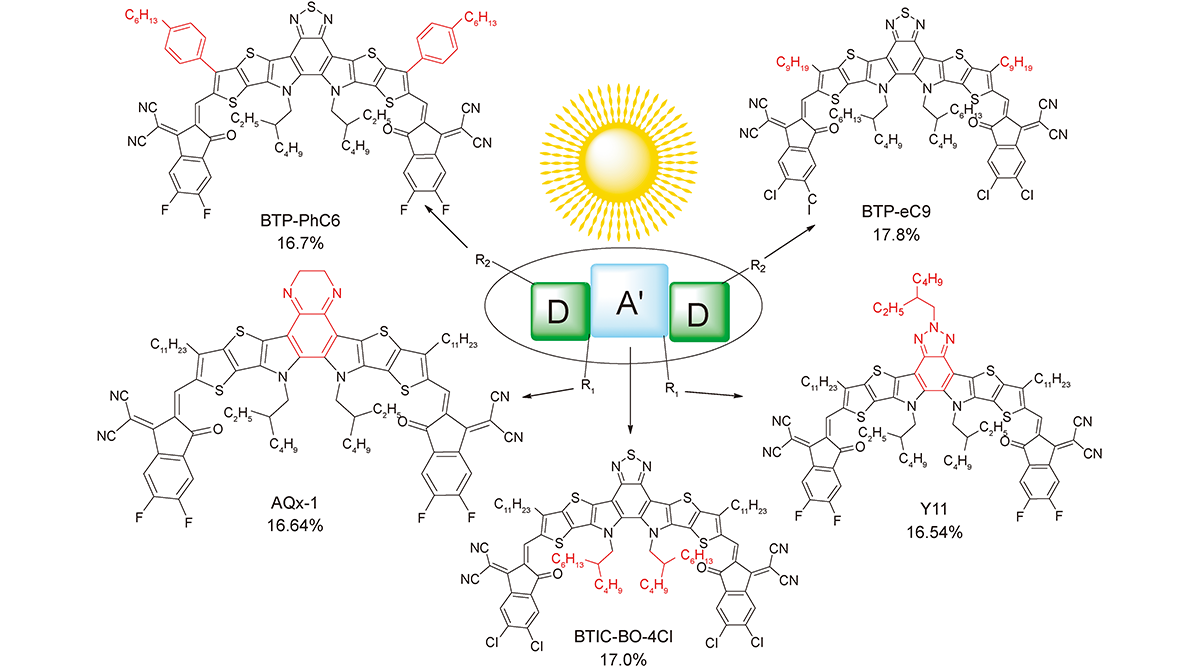
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |