 PDF(10199 KB)
PDF(10199 KB)


电解质在超级电容器中的应用
易锦馨, 霍志鹏, Abdullah M. Asiri, Khalid A. Alamry, 李家星
化学进展 ›› 2018, Vol. 30 ›› Issue (11) : 1624-1633.
 PDF(10199 KB)
PDF(10199 KB)
 PDF(10199 KB)
PDF(10199 KB)
电解质在超级电容器中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Development and Application of Electrolytes in Supercapacitors
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}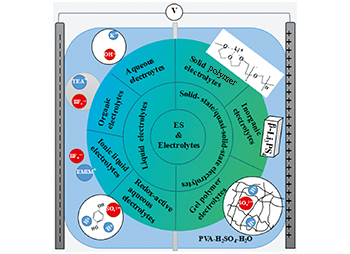
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |