 PDF(8403 KB)
PDF(8403 KB)


 PDF(8403 KB)
PDF(8403 KB)
 PDF(8403 KB)
PDF(8403 KB)
制备固体氧化物燃料电池中电解质薄膜的电泳沉积法
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Electrophoretic Deposition in the Preparation of Electrolyte Thin Films for Solid Oxide Fuel Cells
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}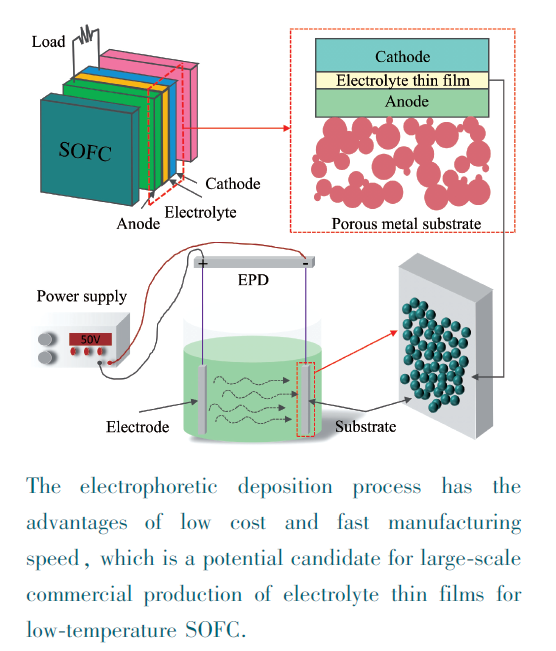
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |