 PDF(8693 KB)
PDF(8693 KB)


 PDF(8693 KB)
PDF(8693 KB)
 PDF(8693 KB)
PDF(8693 KB)
邻菲罗啉类配体在铁系元素催化反应中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Progresses of 1,10-Phenanthroline Type Ligands in Fe/Co/Ni Catalysis
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}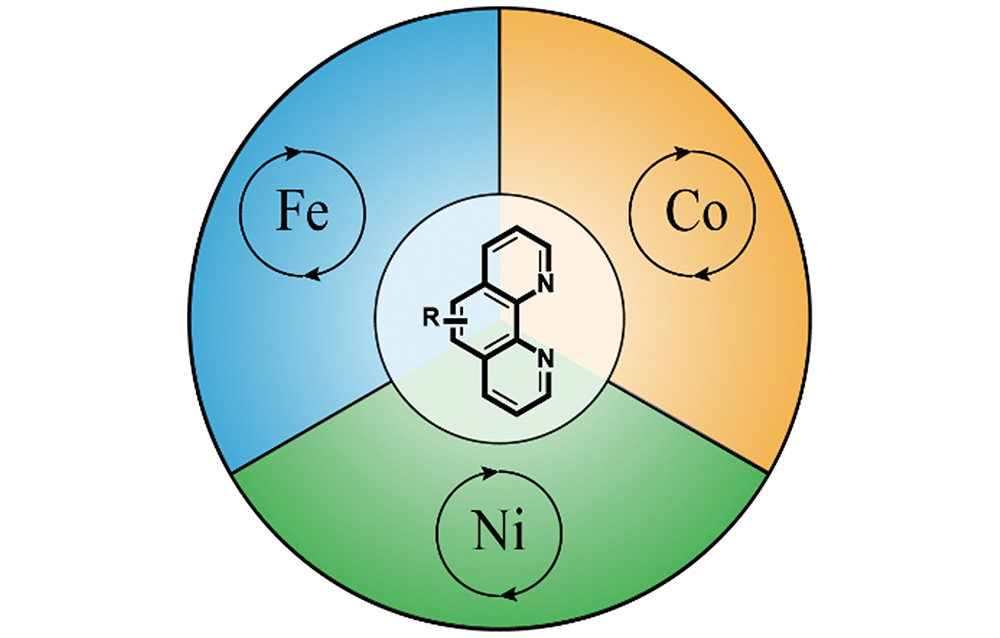
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |