 PDF(11629 KB)
PDF(11629 KB)


 PDF(11629 KB)
PDF(11629 KB)
 PDF(11629 KB)
PDF(11629 KB)
从铜离子、酸中心与铝分布的关系分析不同模板剂制备Cu-SSZ-13的NH3-SCR性能
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Relation Among Cu2+, Brønsted Acid Sites and Framework Al Distribution: NH3-SCR Performance of Cu-SSZ-13 Formed with Different Templates
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}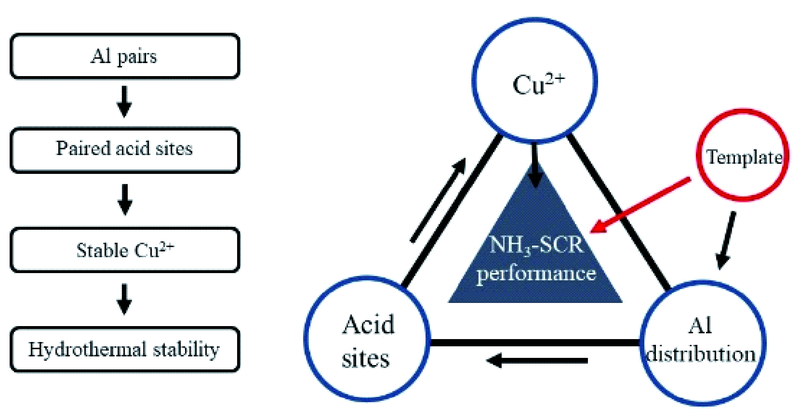
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |