 PDF(13792 KB)
PDF(13792 KB)


 PDF(13792 KB)
PDF(13792 KB)
 PDF(13792 KB)
PDF(13792 KB)
仿衣壳结构的巨大中空超分子纳米容器的多组分自组装构筑及其功能
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Capsid-Inspired Multi-Component Self-Assembly of Nanocontainers: Structure, Functionalization, and Applications
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}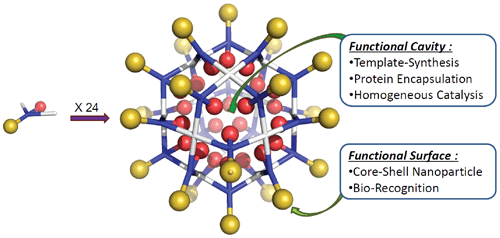
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |