| Electrode name | Electrolytic cell type | Electrolyte | CO2 flow velocity | Reference electrode | iR compensation | Ref |
|---|---|---|---|---|---|---|
| Cu hetero-interface electrode | H-type cell | 0.1 M KHCO3(CO2 saturated,30 mL) | 20 mL/min(750 r/min) | Ag/AgCl(Converted to RHE) | 85% | 26 |
| CuOx soft-landed catalyst electrode | H-type cell and gas diffusion electrode | 0.1 M or 1 M KHCO3(CO2 saturated,circulation volume 4~5 mL) | H-cell:persistent bubbling;Flow cell:5~7.5 sccm | Ag/AgCl(Leakage type, converted to RHE) | -(Not explicitly stated in the text) | 24 |
| Cu-polymer composite electrode | Flow cell | 1 M KOH(0.7 mL/min) | 12 sccm | Ag/AgCl(3 M KCl,converted to RHE) | - | 46 |
| Nanoporous Cu cathode (CO2-triggered break-in) | Zero-Gap MEA Electrolyze + Three-Electrode Half-Cell Testing | half-cell:0.1 M KOH / 0.5 M KHCO3;MEA:Anode 0.05 M KOH | MEA: cathode 50 sccm wet CO2, anode 15 sccm KHCO3 | Half-cell:Hg/HgO;MEA:Full Battery Test | - | 47 |
| Highly porous Cu catalyst | Microfluidic flow Electrolyze | 1 M KOH、1 M KHCO3、1 M KCl、1 M K2SO4 | - | RHE | - | 48 |
| Porous Zn catalyst electrode | H-type cell and gas diffusion electrode | H-cell: 0.1 M KHCO3;Flow-cell: 1.0 M KHCO3 or 1.0 M KOH | H-cell: 21 mL/min;Flow-cell: 40 mL/min | Ag/AgCl(3 M NaCl,converted to RHE) | 85% | 4 |
| AgSn-SnO2 nanosheet electrode | H-type cell and gas diffusion electrode | H-cell: 0.5 M KHCO3;Flow-cell: 1.0 M KOH;Solid electrolyte: Anode 0.1 M H2SO4,Cathode CO2 gas | H-cell: 20 sccm;Flow cell: 20 sccm;Solid: 30 sccm | H-cell/Flow cell: Ag/AgCl;Solid: two-electrode system | - | 18 |
| M-Pc/Buckypaper composite electrode | H-type cell | 0.5 M KHCO3(CO2/Ar saturated) | 15 mL/min(20 min) | Ag/AgCl(saturated KCl,Luggin capillaries) | 85% (positive feedback) | 60 |
| Biomass-derived carbon aerogel electrode | H-type cell | 0.1 M KHCO3(CO2 saturated,pH≈6.8) | 10 mL/min(30 min) | Ag/AgCl(saturated KCl,Luggin capillaries) | 85% (positive feedback) | 23 |
| 3D printed metal-free carbon electrode | H-type cell | 0.1 M KHCO3(CO2 saturated) | Persistent bubbling | SCE(saturated calomel electrode) | - | 21 |
| 3D printed fluoropolymer GDL electrode | Flow cell | 1 M KHCO3 | 50 sccm | Ag/AgCl(converted to RHE) | - | 13 |
| Imidazolium-functionalized GC electrode | Liquid-phase three-electrode cell + flow-through electrolytic cell (GDE) | Liquid phase:0.5 M [EMIM][BF4]/[PF₆];Flow: Acidic aqueous solution | - | Ag/AgCl(Complemented by Fc⁺/Fc internal markers) | - | 12 |
| Bi@C/Si nanowire photocathode | H-type photoelectrochemical cell (quartz cell, separated by Nafion 117 membrane) | 0.1 M KHCO3(CO2 saturated) | 99.99% CO2, 30 min | Ag/AgCl | - | 9 |
| CNT/Graphene-PPS flexible film electrode | Three-electrode system | 0.5 M Na2SO4(pH≈6)0.5 M PBS(pH 7, 8) | - | SCE(converted to RHE) | - | 20 |
| 3D Graphene-CNT hybrid with Co-MnO NPs | Three-electrode system | 0.1 M KOH 0.1 M H2SO4 | - | RHE | - | 22 |
| Ni-Co sulfide/graphene-CNT composite electrode | Three-electrode system | 6 M KOH | - | Hg/HgO | - | 65 |
 Fig. 1 Electrode configurations of Cu-based materials: (A) Catalytic mechanism of Cu electrode. (B) Scanning electron microscope images (SEM) of 0.5-UiO/Cu. (C) SEM and EDS elemental mapping of cross-section. (D) FE vs. potential of CO2RR and HER products[
Fig. 1 Electrode configurations of Cu-based materials: (A) Catalytic mechanism of Cu electrode. (B) Scanning electron microscope images (SEM) of 0.5-UiO/Cu. (C) SEM and EDS elemental mapping of cross-section. (D) FE vs. potential of CO2RR and HER products[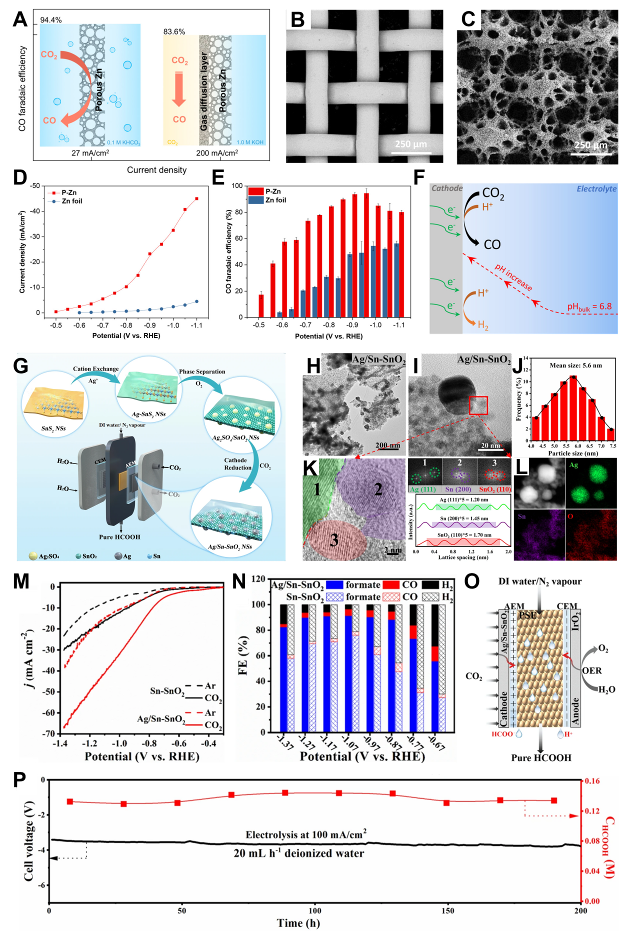 Fig. 2 Electrode configurations of Zn, Ag, and Sn-based materials: (A) Porous Zn electrode can convert CO2 to CO at -0.95 V (relative to the RHE electrode) with high FE (∼95%) and current density (27 mA cm−2). (B) SEM images of Cu lattice and (C) P-Zn. (D) Potential-dependent current densities of P-Zn and Zn foils. (E) FE of CO. (F) Schematic representation of the local pH effect in CO2RR[
Fig. 2 Electrode configurations of Zn, Ag, and Sn-based materials: (A) Porous Zn electrode can convert CO2 to CO at -0.95 V (relative to the RHE electrode) with high FE (∼95%) and current density (27 mA cm−2). (B) SEM images of Cu lattice and (C) P-Zn. (D) Potential-dependent current densities of P-Zn and Zn foils. (E) FE of CO. (F) Schematic representation of the local pH effect in CO2RR[ Fig. 4 Graphene and carbon nanotube thin-film electrode constructs: (A) Synthesis procedures of RGOL@PPS/CNT+RGO thin films. (B, C) SEM images of Cu2O nanocrystals on RGOL@PPS/CNT+RGO thin films at 15 s and (D, E) 90 s electrodeposition times. (F) Polarization curves of (PPS/CNT+RGO)-Cu2O, RGOL@PPS/CNT+RGO and PPS/CNT+RGO substrates with polarization curves[
Fig. 4 Graphene and carbon nanotube thin-film electrode constructs: (A) Synthesis procedures of RGOL@PPS/CNT+RGO thin films. (B, C) SEM images of Cu2O nanocrystals on RGOL@PPS/CNT+RGO thin films at 15 s and (D, E) 90 s electrodeposition times. (F) Polarization curves of (PPS/CNT+RGO)-Cu2O, RGOL@PPS/CNT+RGO and PPS/CNT+RGO substrates with polarization curves[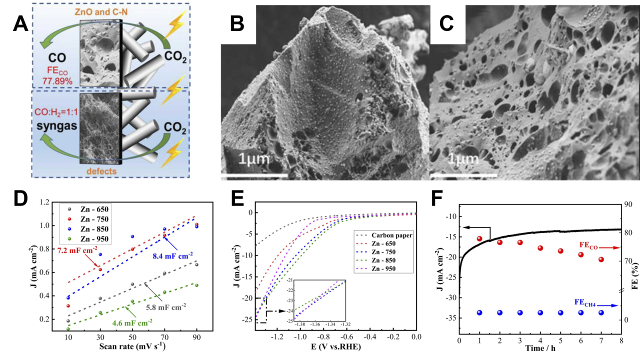 Fig. 5 Carbon aerogel versus carbon foam electrode constructs: (A) Schematic representation of the active sites of Zn-750 and Zn-950. (B) SEM images of Zn-650 and (C) Zn-750. (D) ECSA curves and (E) LSV curves of Zn-T. (F) FE stability of Zn-750 after 8 h of continuous operation at −1.0 V vs. RHE[
Fig. 5 Carbon aerogel versus carbon foam electrode constructs: (A) Schematic representation of the active sites of Zn-750 and Zn-950. (B) SEM images of Zn-650 and (C) Zn-750. (D) ECSA curves and (E) LSV curves of Zn-T. (F) FE stability of Zn-750 after 8 h of continuous operation at −1.0 V vs. RHE[ Fig. 6 Emerging structures with 3D printed electrodes: (A) 3D printed electrodes with different shapes and optimal 3Dp-PNCE catalytic electrodes. (B) TEM images of the optimal 3Dp-PNCE. (C) Comparison of current densities on 3Dp-CE, 3Dp-NCE, and 3Dp-PNCE. (D) CO2RR stability test of 3Dp-PNCE at −0.7 V[
Fig. 6 Emerging structures with 3D printed electrodes: (A) 3D printed electrodes with different shapes and optimal 3Dp-PNCE catalytic electrodes. (B) TEM images of the optimal 3Dp-PNCE. (C) Comparison of current densities on 3Dp-CE, 3Dp-NCE, and 3Dp-PNCE. (D) CO2RR stability test of 3Dp-PNCE at −0.7 V[ Fig. 7 Surface and interface engineering: (A) Schematic representation of CO2 conversion to formate mediated on a GC electrode in a liquid-phase electrolyzer and CO2RR mediated on a GDE modified by [EMIM]+ layer in a gas-phase electrolyzer. (B) Conformational and chemical characterization of pristine GC cathode and modified GC cathode obtained by immobilization of imidazolium cation by SEM and WCA. (C) On IM+EE/GDE cathode with 9 successive electrolysis at different current densities, respectively. (D) Variation of the average full-cell energy efficiency and EC of formate generation with applied current density on bare carbon GDE and IM+EE/GDE, respectively[
Fig. 7 Surface and interface engineering: (A) Schematic representation of CO2 conversion to formate mediated on a GC electrode in a liquid-phase electrolyzer and CO2RR mediated on a GDE modified by [EMIM]+ layer in a gas-phase electrolyzer. (B) Conformational and chemical characterization of pristine GC cathode and modified GC cathode obtained by immobilization of imidazolium cation by SEM and WCA. (C) On IM+EE/GDE cathode with 9 successive electrolysis at different current densities, respectively. (D) Variation of the average full-cell energy efficiency and EC of formate generation with applied current density on bare carbon GDE and IM+EE/GDE, respectively[