Fig. 2 Electrode configurations of Zn, Ag, and Sn-based materials: (A) Porous Zn electrode can convert CO2 to CO at -0.95 V (relative to the RHE electrode) with high FE (∼95%) and current density (27 mA cm−2). (B) SEM images of Cu lattice and (C) P-Zn. (D) Potential-dependent current densities of P-Zn and Zn foils. (E) FE of CO. (F) Schematic representation of the local pH effect in CO2RR[4]. (G) Schematic representation of the synthesis process of Ag/Sn-SnO2 nanosheets electrocatalyzing the conversion of CO2 to pure HCOOH solution. (H, I) Typical TEM images of Ag/Sn-SnO2 NSs. (J) Particle size distribution of Sn-SnO2 in Ag/Sn-SnO2 NSs. (K) Particle size distribution of Ag/HRTEM images and corresponding FFT maps of Sn-SnO2 NSs with integrated pixel intensities of Ag, Sn, and SnO2. (L) HAADF-STEM images and EDX mapping images of Ag/Sn-SnO2 NSs. (M) Electrocatalytic LSV curves. (N) FEs of formate CO and H2. (O) Reduction of CO2 in PSE cell to pure HCOOH with accompanying OER reaction. (P) Concentration of HCOOH under deionized water as a diffusion carrier, and the FE of the corresponding HCOOH[18]
Other figure/table from this article
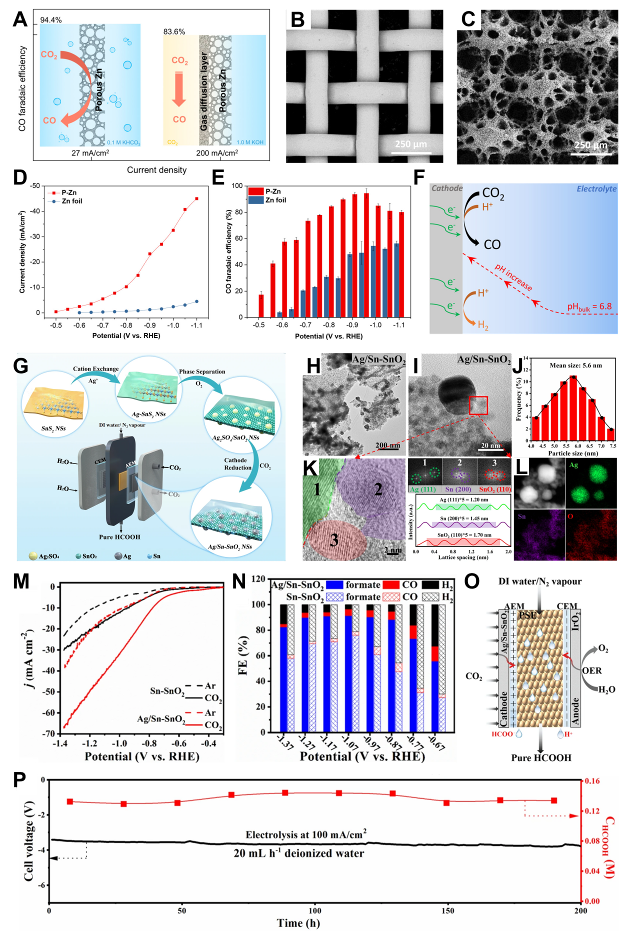
 Fig. 1 Electrode configurations of Cu-based materials: (A) Catalytic mechanism of Cu electrode. (B) Scanning electron microscope images (SEM) of 0.5-UiO/Cu. (C) SEM and EDS elemental mapping of cross-section. (D) FE vs. potential of CO2RR and HER products[
Fig. 1 Electrode configurations of Cu-based materials: (A) Catalytic mechanism of Cu electrode. (B) Scanning electron microscope images (SEM) of 0.5-UiO/Cu. (C) SEM and EDS elemental mapping of cross-section. (D) FE vs. potential of CO2RR and HER products[ Fig. 4 Graphene and carbon nanotube thin-film electrode constructs: (A) Synthesis procedures of RGOL@PPS/CNT+RGO thin films. (B, C) SEM images of Cu2O nanocrystals on RGOL@PPS/CNT+RGO thin films at 15 s and (D, E) 90 s electrodeposition times. (F) Polarization curves of (PPS/CNT+RGO)-Cu2O, RGOL@PPS/CNT+RGO and PPS/CNT+RGO substrates with polarization curves[
Fig. 4 Graphene and carbon nanotube thin-film electrode constructs: (A) Synthesis procedures of RGOL@PPS/CNT+RGO thin films. (B, C) SEM images of Cu2O nanocrystals on RGOL@PPS/CNT+RGO thin films at 15 s and (D, E) 90 s electrodeposition times. (F) Polarization curves of (PPS/CNT+RGO)-Cu2O, RGOL@PPS/CNT+RGO and PPS/CNT+RGO substrates with polarization curves[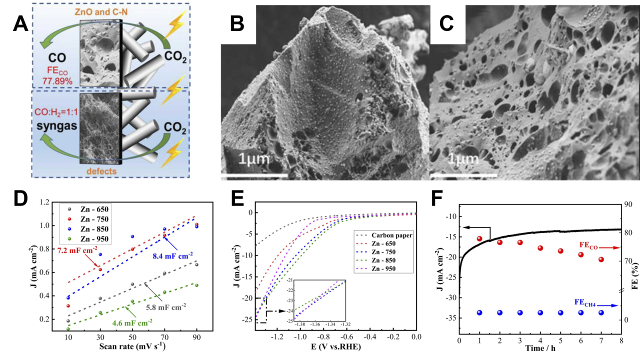 Fig. 5 Carbon aerogel versus carbon foam electrode constructs: (A) Schematic representation of the active sites of Zn-750 and Zn-950. (B) SEM images of Zn-650 and (C) Zn-750. (D) ECSA curves and (E) LSV curves of Zn-T. (F) FE stability of Zn-750 after 8 h of continuous operation at −1.0 V vs. RHE[
Fig. 5 Carbon aerogel versus carbon foam electrode constructs: (A) Schematic representation of the active sites of Zn-750 and Zn-950. (B) SEM images of Zn-650 and (C) Zn-750. (D) ECSA curves and (E) LSV curves of Zn-T. (F) FE stability of Zn-750 after 8 h of continuous operation at −1.0 V vs. RHE[ Fig. 6 Emerging structures with 3D printed electrodes: (A) 3D printed electrodes with different shapes and optimal 3Dp-PNCE catalytic electrodes. (B) TEM images of the optimal 3Dp-PNCE. (C) Comparison of current densities on 3Dp-CE, 3Dp-NCE, and 3Dp-PNCE. (D) CO2RR stability test of 3Dp-PNCE at −0.7 V[
Fig. 6 Emerging structures with 3D printed electrodes: (A) 3D printed electrodes with different shapes and optimal 3Dp-PNCE catalytic electrodes. (B) TEM images of the optimal 3Dp-PNCE. (C) Comparison of current densities on 3Dp-CE, 3Dp-NCE, and 3Dp-PNCE. (D) CO2RR stability test of 3Dp-PNCE at −0.7 V[ Fig. 7 Surface and interface engineering: (A) Schematic representation of CO2 conversion to formate mediated on a GC electrode in a liquid-phase electrolyzer and CO2RR mediated on a GDE modified by [EMIM]+ layer in a gas-phase electrolyzer. (B) Conformational and chemical characterization of pristine GC cathode and modified GC cathode obtained by immobilization of imidazolium cation by SEM and WCA. (C) On IM+EE/GDE cathode with 9 successive electrolysis at different current densities, respectively. (D) Variation of the average full-cell energy efficiency and EC of formate generation with applied current density on bare carbon GDE and IM+EE/GDE, respectively[
Fig. 7 Surface and interface engineering: (A) Schematic representation of CO2 conversion to formate mediated on a GC electrode in a liquid-phase electrolyzer and CO2RR mediated on a GDE modified by [EMIM]+ layer in a gas-phase electrolyzer. (B) Conformational and chemical characterization of pristine GC cathode and modified GC cathode obtained by immobilization of imidazolium cation by SEM and WCA. (C) On IM+EE/GDE cathode with 9 successive electrolysis at different current densities, respectively. (D) Variation of the average full-cell energy efficiency and EC of formate generation with applied current density on bare carbon GDE and IM+EE/GDE, respectively[