
 Fig. 2 (A) Schematic illustration of the proposed mechanism for the formation of Pt-CNSs/rGO nanohybrids. (B) TEM image of Pt-CNSs, insets: (a) HRTEM image and (b) FFT pattern of Pt-CNSs. (C) Typical TEM images of Pt-CNSs/rGO nanohybrids. (D) HER polarization curves of Pt-CNSs/rGO nanohybrids and Pt-CNSs in N2-saturated 0.5 M H2SO4 solution at a scan rate of 5 mV/s and rotation rate of 1000 r/min. The top-right inset shows the corresponding Tafel plots for Pt-CNSs/rGO nanohybrids and Pt-CNSs. (E) Electrochemical impedance spectra of Pt-CNSs/rGO nanohybrids and Pt-CNSs[
Fig. 2 (A) Schematic illustration of the proposed mechanism for the formation of Pt-CNSs/rGO nanohybrids. (B) TEM image of Pt-CNSs, insets: (a) HRTEM image and (b) FFT pattern of Pt-CNSs. (C) Typical TEM images of Pt-CNSs/rGO nanohybrids. (D) HER polarization curves of Pt-CNSs/rGO nanohybrids and Pt-CNSs in N2-saturated 0.5 M H2SO4 solution at a scan rate of 5 mV/s and rotation rate of 1000 r/min. The top-right inset shows the corresponding Tafel plots for Pt-CNSs/rGO nanohybrids and Pt-CNSs. (E) Electrochemical impedance spectra of Pt-CNSs/rGO nanohybrids and Pt-CNSs[ Fig. 3 (A) Schematic solvothermal synthesis with GO sheets to afford the MoS2/rGO hybrid. (B) SEM and (inset) TEM images of the MoS2/RGO hybrid. (C) Schematic solvothermal synthesis without any GO sheets, resulting in large, free MoS2 particles. (D) SEM and (inset) TEM images of the free particles. (E) TEM image showing folded edges of MoS2 particles on RGO in the hybrid. The inset shows a magnified image of the folded edge of a MoS2 nanoparticle. (F) HRTEM image showing nanosized MoS2 with highly exposed edges stacked on a RGO sheet. Polarization curves (G) and corresponding Tafel plots (H) of different electrocatalysts[
Fig. 3 (A) Schematic solvothermal synthesis with GO sheets to afford the MoS2/rGO hybrid. (B) SEM and (inset) TEM images of the MoS2/RGO hybrid. (C) Schematic solvothermal synthesis without any GO sheets, resulting in large, free MoS2 particles. (D) SEM and (inset) TEM images of the free particles. (E) TEM image showing folded edges of MoS2 particles on RGO in the hybrid. The inset shows a magnified image of the folded edge of a MoS2 nanoparticle. (F) HRTEM image showing nanosized MoS2 with highly exposed edges stacked on a RGO sheet. Polarization curves (G) and corresponding Tafel plots (H) of different electrocatalysts[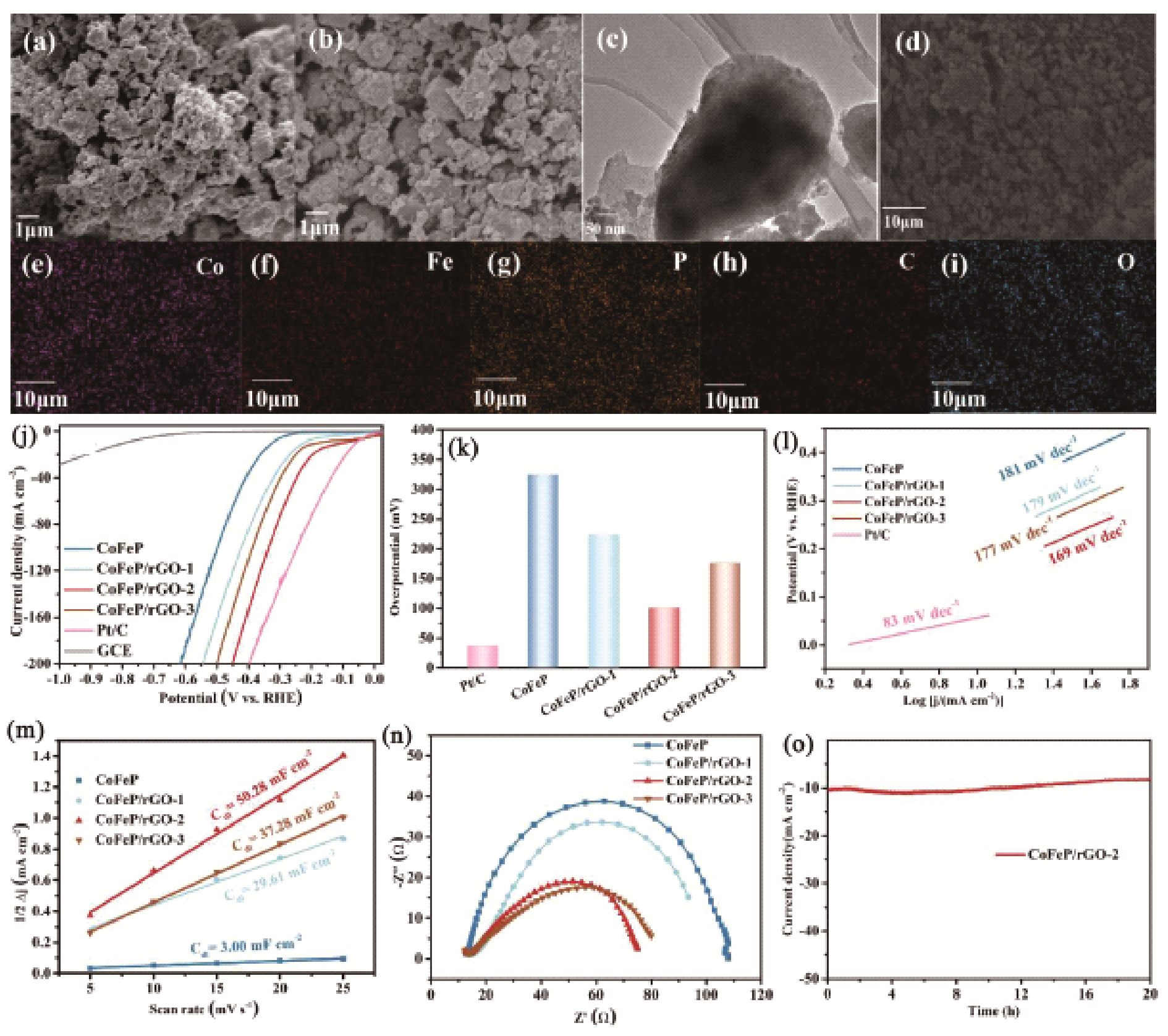 Fig. 5 SEM images of (a) CoFeP and (b) CoFeP/rGO, (d) TEM image and corresponding elemental mapping images (e~i) of CoFeP/rGO. HER performance of CoFeP and CoFeP/rGO composites in the 0.5 M H2SO4 solution: (j) HER polarization curves; (k) Overpotentials; (l) Tafel plots; (m) Capacitive current densities; (n) Nyquist plots; (o) i-t curves of CoFeP/rGO at potential of 0.076 V vs RHE[
Fig. 5 SEM images of (a) CoFeP and (b) CoFeP/rGO, (d) TEM image and corresponding elemental mapping images (e~i) of CoFeP/rGO. HER performance of CoFeP and CoFeP/rGO composites in the 0.5 M H2SO4 solution: (j) HER polarization curves; (k) Overpotentials; (l) Tafel plots; (m) Capacitive current densities; (n) Nyquist plots; (o) i-t curves of CoFeP/rGO at potential of 0.076 V vs RHE[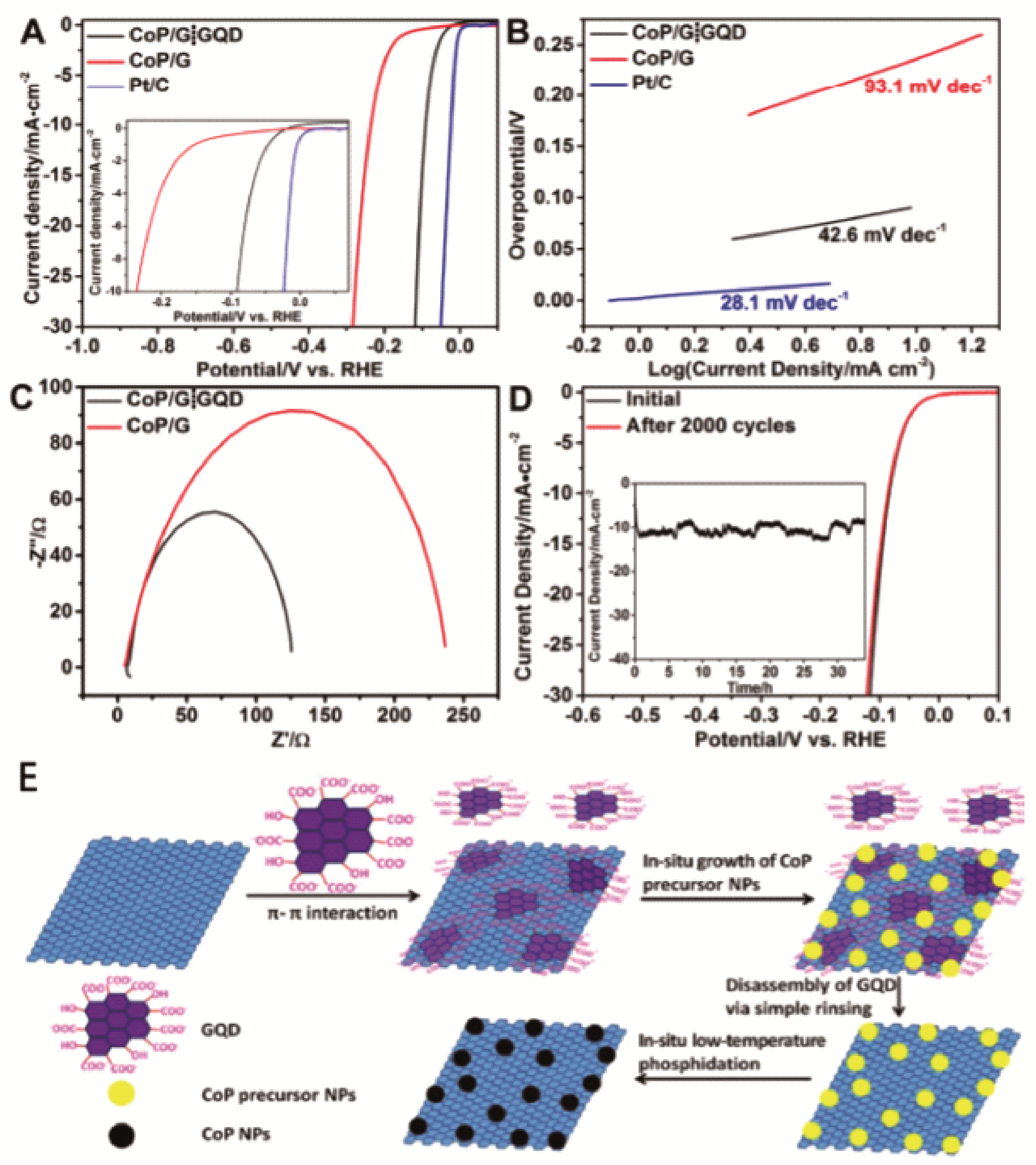 Fig. 6 LSV curves (A) and Tafel plots (B) of CoP/G⁞GQD, CoP/G, and commercial Pt/C; (C) Nyquist plots of CoP/G⁞GQD and CoP/G measured at an overpotential of 200 mV in a frequency range from 106 to 1 Hz; (D) LSV curves of CoP/G⁞GQD at a scan rate of 2 mV/s before and after 2000 CV cycles at a scan rate of 100 mV/s between -0.17 and +0.01 V. Inset: time dependence of the current density of CoP/ G⁞GQD at an overpotential of 91.3 mV; (E) schematic illustration of the synthesis process of CoP/G⁞GQD[
Fig. 6 LSV curves (A) and Tafel plots (B) of CoP/G⁞GQD, CoP/G, and commercial Pt/C; (C) Nyquist plots of CoP/G⁞GQD and CoP/G measured at an overpotential of 200 mV in a frequency range from 106 to 1 Hz; (D) LSV curves of CoP/G⁞GQD at a scan rate of 2 mV/s before and after 2000 CV cycles at a scan rate of 100 mV/s between -0.17 and +0.01 V. Inset: time dependence of the current density of CoP/ G⁞GQD at an overpotential of 91.3 mV; (E) schematic illustration of the synthesis process of CoP/G⁞GQD[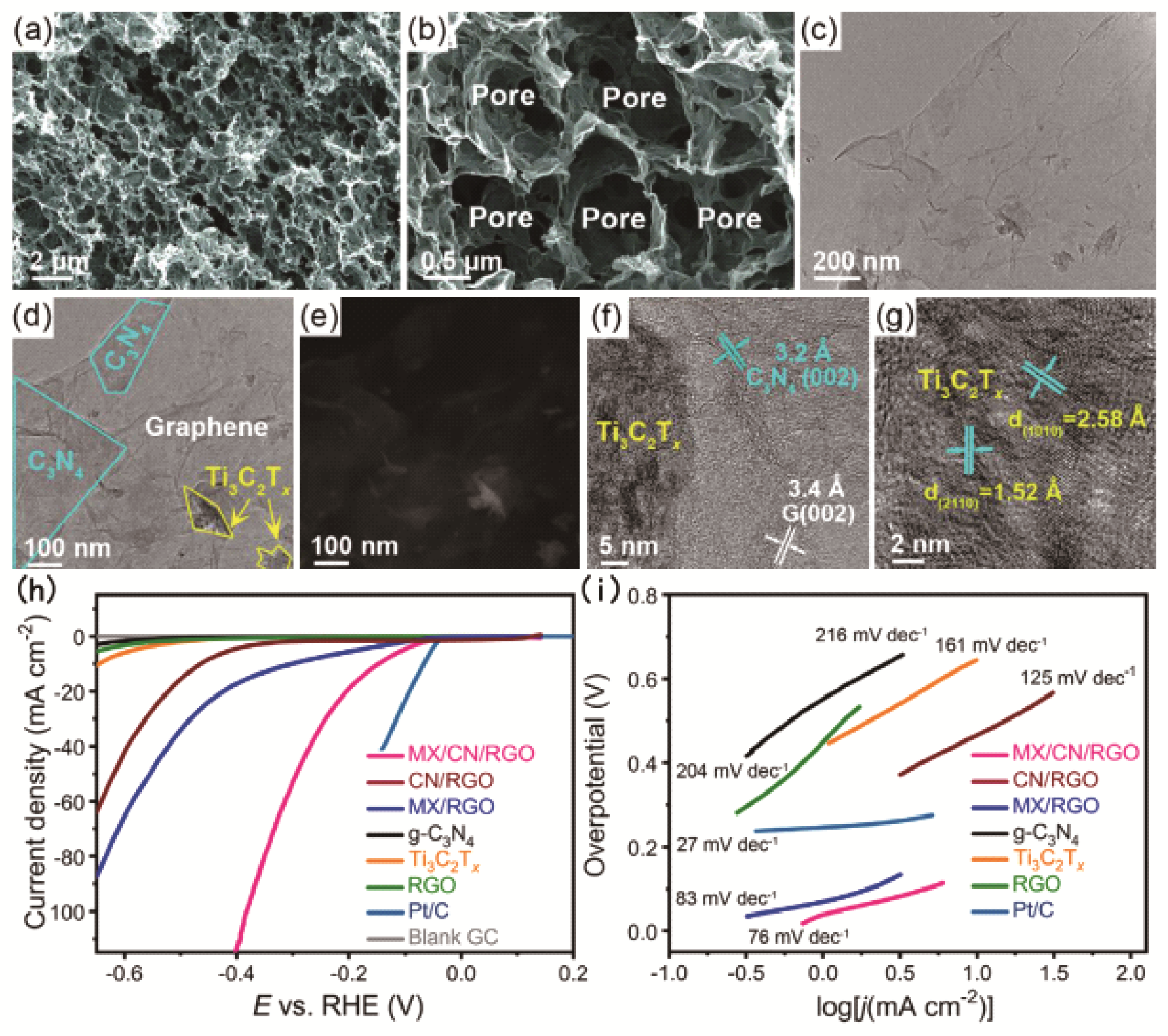 Fig. 7 Morphological and microstructural analysisof the 3D MX/CN/RGO nanoarchitecture. Representative (a, b) FE-SEM, (c, d) TEM, (e) HAADF-STEM images reveal the successful integration of Ti3C2Tx, g-C3N4 nanosheets and graphene into a 3D interconnected framework; (f, g) HR-TEM images disclose the lattice fringes of Ti3C2Tx and g-C3N4 nanosheets; (h) LSV polarization curves and (i) the corresponding Tafel plots[
Fig. 7 Morphological and microstructural analysisof the 3D MX/CN/RGO nanoarchitecture. Representative (a, b) FE-SEM, (c, d) TEM, (e) HAADF-STEM images reveal the successful integration of Ti3C2Tx, g-C3N4 nanosheets and graphene into a 3D interconnected framework; (f, g) HR-TEM images disclose the lattice fringes of Ti3C2Tx and g-C3N4 nanosheets; (h) LSV polarization curves and (i) the corresponding Tafel plots[ Fig. 8 (a, b) HRTEM images of CoNi@NC, showing the graphene shells and encapsulated metal nanoparticles. (c) Schematic illustration of the CoNi@NC structure. (d) Statistical analysis of the number of layers in the graphene shells encapsulating the metal nanoparticles in CoNi@NC. (e~h) HAADF-STEM image and corresponding elemental mapping images of CoNi@NC. (i) Gibbs free energy (ΔG) profile of the HER on various catalysts. (j) Volcano plot of the polarized current (i0) versus ΔG(H*) for a CoNi cluster, CoNi@C, and an N-doped graphene shell (Ncarbon)[
Fig. 8 (a, b) HRTEM images of CoNi@NC, showing the graphene shells and encapsulated metal nanoparticles. (c) Schematic illustration of the CoNi@NC structure. (d) Statistical analysis of the number of layers in the graphene shells encapsulating the metal nanoparticles in CoNi@NC. (e~h) HAADF-STEM image and corresponding elemental mapping images of CoNi@NC. (i) Gibbs free energy (ΔG) profile of the HER on various catalysts. (j) Volcano plot of the polarized current (i0) versus ΔG(H*) for a CoNi cluster, CoNi@C, and an N-doped graphene shell (Ncarbon)[