 PDF(6715 KB)
PDF(6715 KB)


 PDF(6715 KB)
PDF(6715 KB)
 PDF(6715 KB)
PDF(6715 KB)
金属有机框架纳米酶在临床检测中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Metal-Organic Framework-Based Nanozymes for Clinical Applications
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}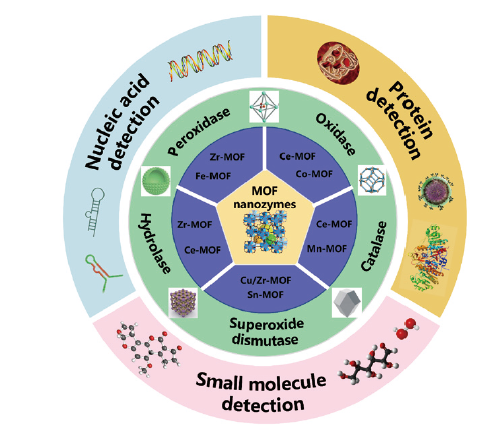
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |