 PDF(15833 KB)
PDF(15833 KB)


 PDF(15833 KB)
PDF(15833 KB)
 PDF(15833 KB)
PDF(15833 KB)
金属有机框架材料在质子交换膜燃料电池中的潜在应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Potential Applications of Metal Organic Framework-Based Materials for Proton Exchange Membrane Fuel Cells
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}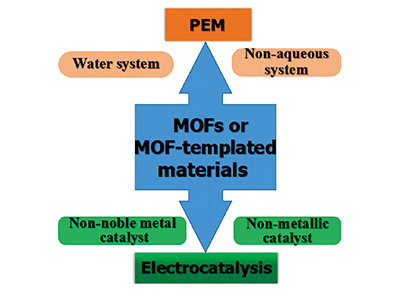
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |