 PDF(17329 KB)
PDF(17329 KB)


 PDF(17329 KB)
PDF(17329 KB)
 PDF(17329 KB)
PDF(17329 KB)
过渡金属配合物催化炔烃和亲核试剂的羰化反应
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Carbonylation of Alkynes with Different Nucleophiles Catalyzed By Transition Metal Complexes
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}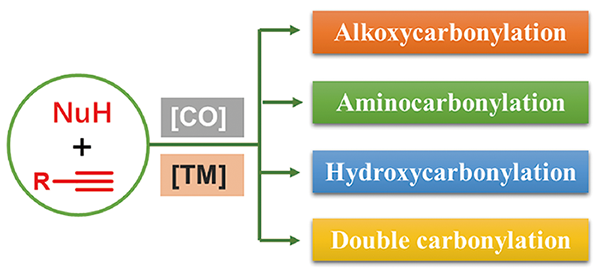
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |