 PDF(5765 KB)
PDF(5765 KB)


 PDF(5765 KB)
PDF(5765 KB)
 PDF(5765 KB)
PDF(5765 KB)
TinO2n-1系列氧化物的特性、制备方法及应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}TinO2n-1 Series Compounds——Properties, Preparation Methods and Applications
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}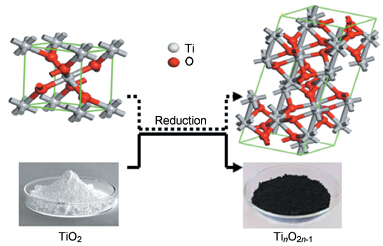
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |