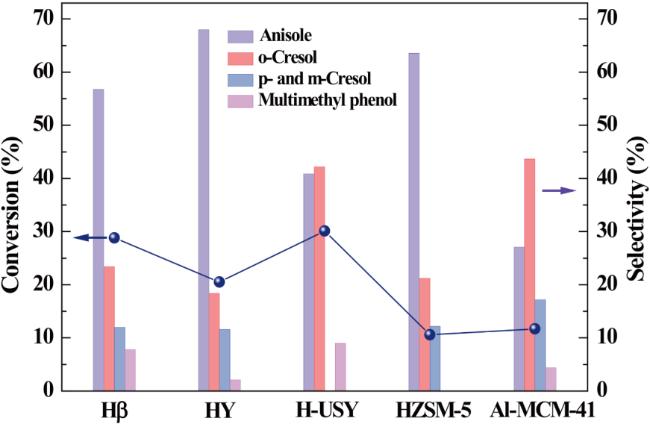
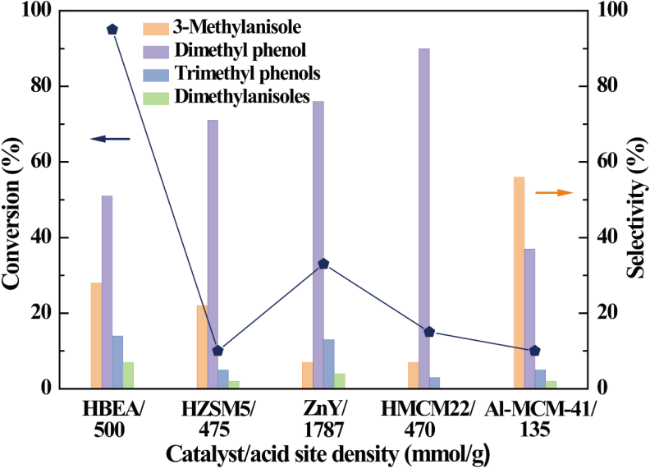
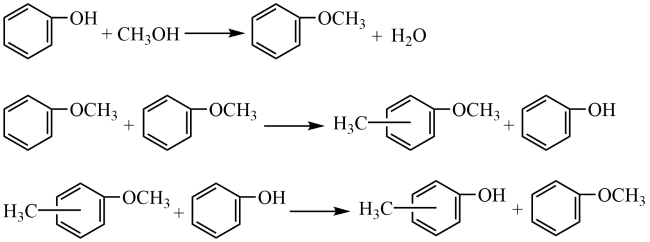
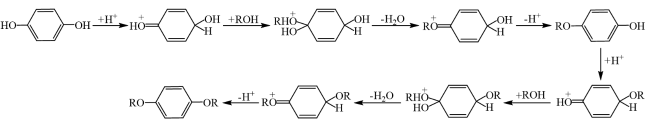
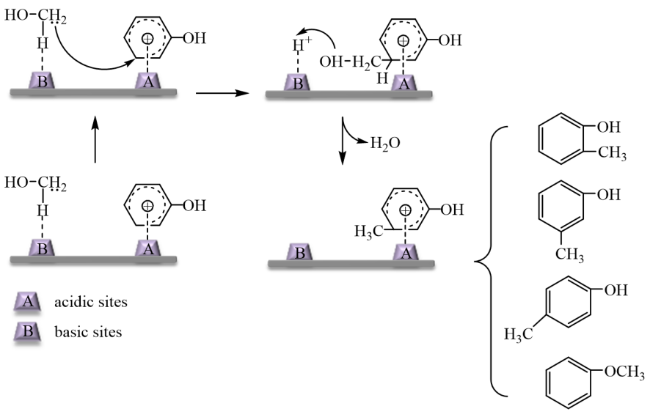
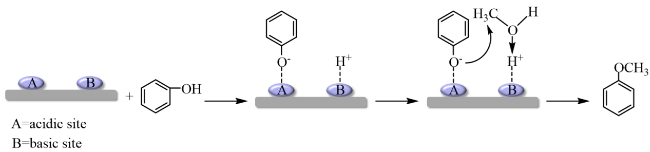
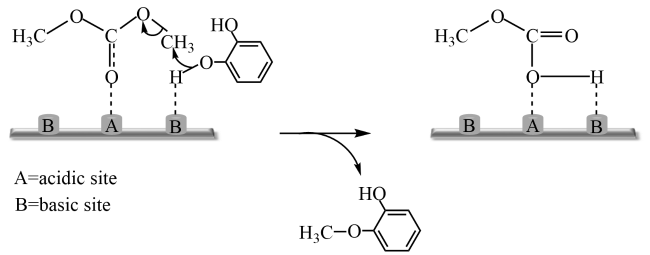
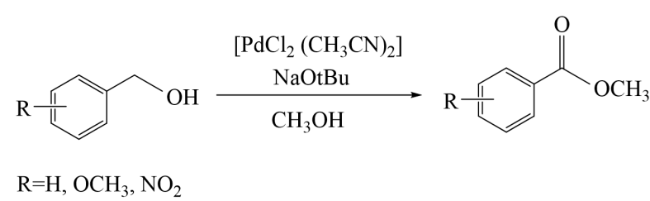
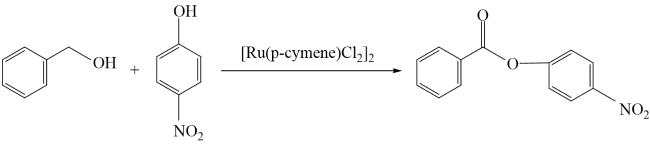
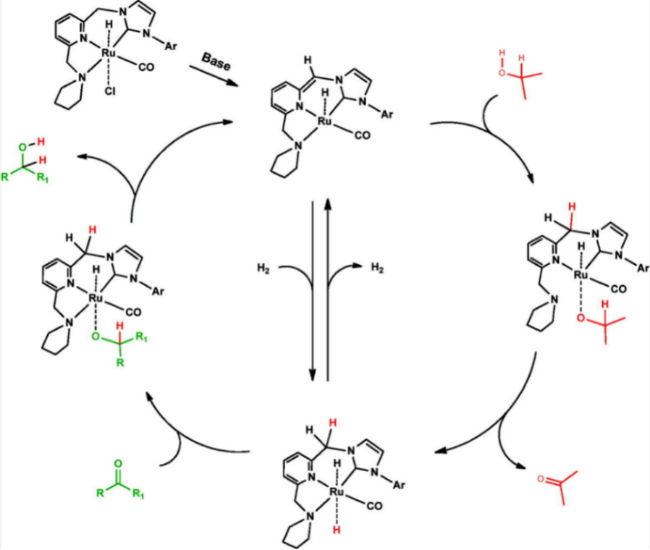
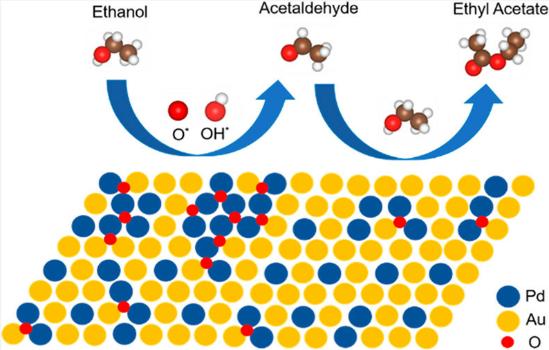
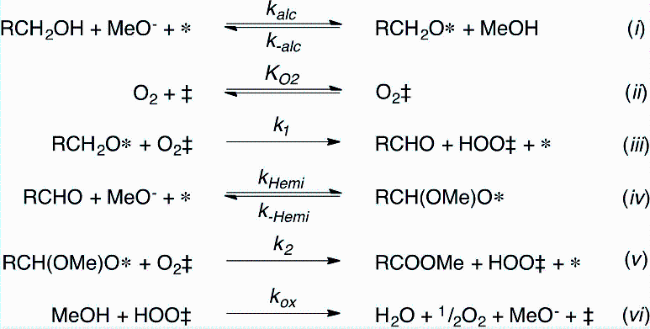
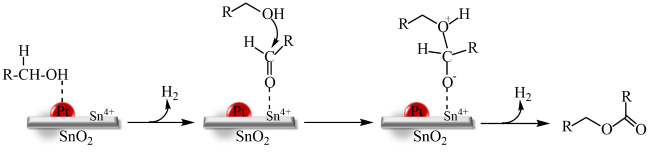
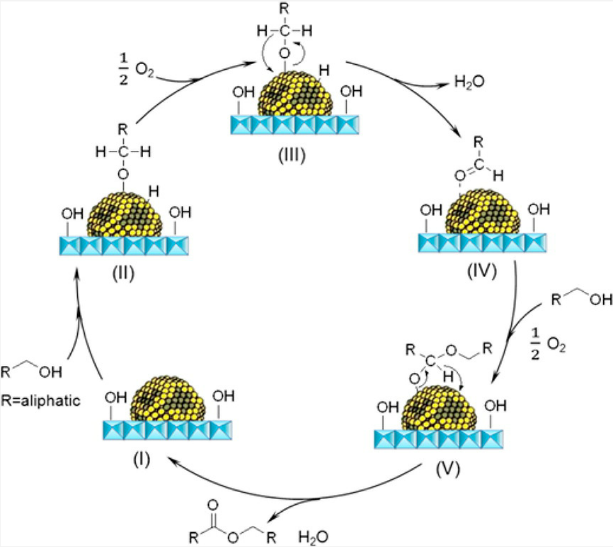
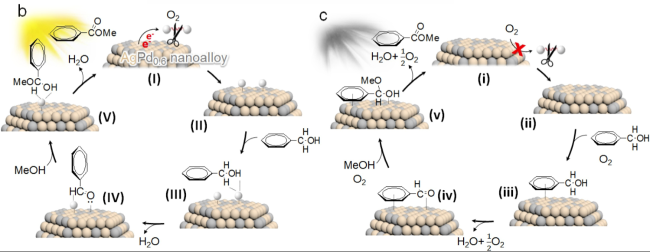
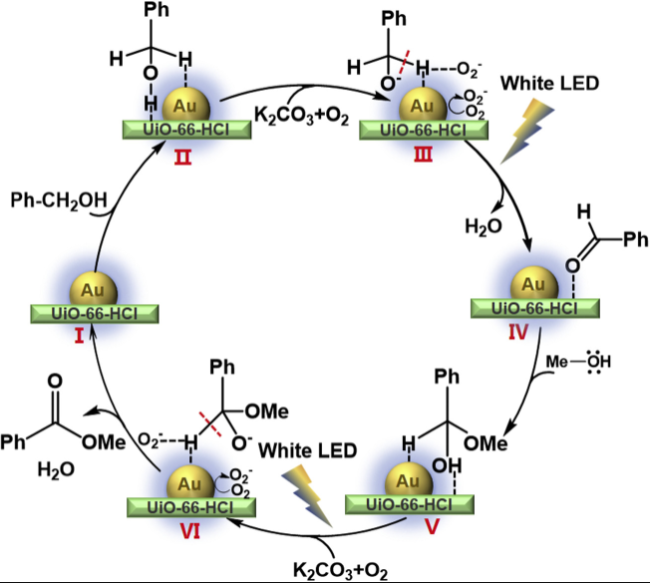
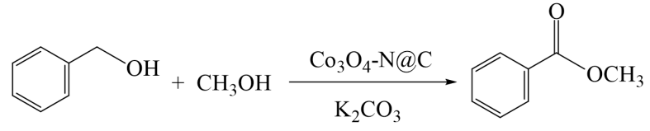In addition, the support can not only be used as an active site for the activation of reactant molecules but also have great effects on the size and surface charge of metal particles. The synergistic effect of the metal and the support drive the reaction and achieve superior catalytic activity in mild reaction conditions. In 2011, Gusevskaya
et al. [59] used mesoporous SiO
2 (HMS) as a support to prepare Au-supported catalysts for the oxidative esterification of benzyl alcohol with methanol. When Au/HMS was used without modification as the catalyst and K
2CO
3 as an additive, the TOF value was 744 h
−1, and the selectivity of methyl benzoate was 48% at 383 K. The catalyst activity obviously increased when the catalyst was modified by Fe, Ce and Ti, and the catalytic activity of Au/HMS-Ce was the best. The TOF was up to 995 h
−1 and the selectivity of methyl benzoate was as high as 94% under the same reaction conditions. It was concluded that when HMS was modified by Fe, Ce and Ti, the smaller Au particle clusters could be achieved, and the surface of these small clusters would become negative charge due to the interaction with oxygen molecules, which could used as Lewis basic sites to activate the reactant molecules. In 2013, Zhaorigetu
et al. [60] synthesized a series of metal oxide-supported Au nanoparticle catalysts for the oxidative esterification of benzyl alcohol with methanol. Compared with Au/TiO
2, Au/CeO
2, Au/Al
2O
3 and Au/ZnO, the catalytic activity of Au/ZrO
2 was the best. When 3 wt% Au/ZrO
2 was used as the catalyst, the conversion of benzyl alcohol was up to 95%, and the selectivity of methyl benzoate was 95% at 303 K for 24 h. It was found that the Au particle size in Au/Al
2O
3 was significantly bigger than the others, and this could be the main reason for the low catalytic activity of Au/Al
2O
3. It can be seen that the support had a great effect on the size of Au particles during the synthesis process, which was crucial to the catalytic activity. In 2014, Han
et al. [61] synthesized a stable porous ionic liquid-hydrogel by the induction of inorganic salts and loading the metal nanoparticles successfully for the first time. This method can prepare metal catalysts with a particle size of less than 1 nm, such as Au/SiO
2, Ru/SiO
2, Pd/Cu-MOF, and Au/PAM. The 1.0 wt% Au/SiO
2 catalyst performed excellent catalytic activity in the oxidative esterification of benzyl alcohol with methanol. The conversion of benzyl alcohol was above 99%, and the methyl ester selectivity was also above 99% when using K
2CO
3 as an additive at 323 K for 24 h. The small metal particle size prepared by the porous ionic liquid-hydrogel one-step method and the porous characteristics of the support were the main reasons for its excellent catalytic activity. The support plays an important role in promoting the adsorption of reactant molecules and the mass transfer of intermediate species. It can be seen that the supported metal catalyst should include two active sites: metal particles and support. The synergistic effect of these two active sites promotes the reaction, and it also proves that Au particles with smaller particle sizes were beneficial to the direct oxidation of alcohol to esters. Li
et al. [62] synthesized a series of Au nanoparticle catalysts using different metal oxides as supports. Catalyst Au/CeO
2 and Au/ZrO
2 performed higher catalytic activity than Au/TiO
2, Au/HT and Au/Al
2O
3 in the oxidative esterification of benzyl alcohol with methanol. When using 2.8 wt% Au/CeO
2 as the catalyst and Cs
2CO
3 as the base additive, the conversion of benzyl alcohol was up to 99.7%, and the selectivity of methyl benzoate was 99.4% at 298 K for 6 h. When using 2.9 wt% Au/ZrO
2 as a catalyst, the benzyl alcohol conversion of 100% and a methyl benzoate selectivity of 99% were achieved at 298 K for 6 h. It was not only because of the different sizes of Au nanoparticles prepared by different supports but also because of the different interactions of support and reactant molecules. The reaction mechanism is shown in
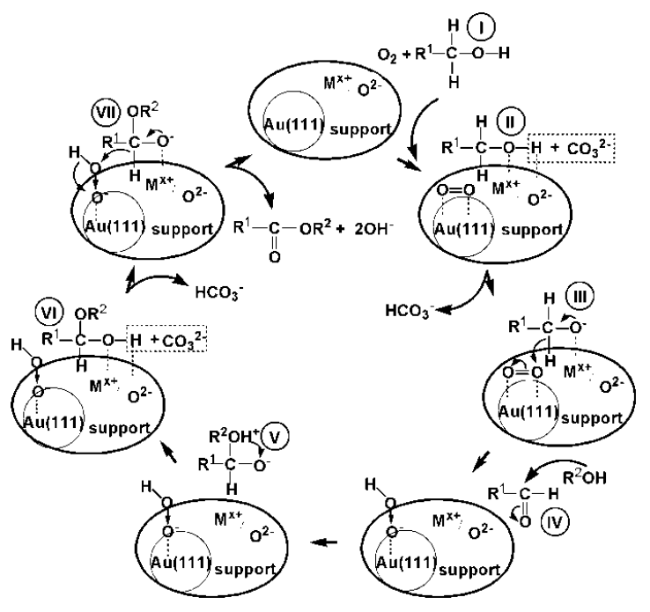
Fig. 16: Firstly, the benzyl alcohol molecule was adsorbed on the surface of the support. The hydrogen atom of the benzyl alcohol hydroxyl interacted with the oxygen atom of the support. The hydroxyl oxygen atom interacted with the metal ion of the support, promoting the cleavage of OH. The desorbed H was captured by the carbonate ion of Cs
2CO
3 to form HCO
3−. At the same time, the oxygen molecule was activated by Au particles to O=O, and then the α-H of alcohol oxygen species was attracted by the oxygen active species O=O, which caused the cleavage of α-H bond to form the intermediate species benzaldehyde. Then, the nucleophilic addition of methanol molecule and benzaldehyde formed a hemiacetal species, and the second α-H of the alcoholic oxygen species was also attracted by the oxygen-active species O=O, leading to the cleavage of the bond. Finally, the product molecule and OH
- were formed. The role of support in the catalytic process was discussed in-depth in this work. In 2023, Ishida
et al. [63] synthesized a gold nanoparticle deposited on the cation and anion-substituted hydroxyapatites (Au/sHAPs) catalyst for the oxidative esterification of octanal or 1-octanol with ethanol to obtain ethyl octanoate. It was found that the metal-support interaction (SMSI) plays an important role in catalytic activity, in which the SMSI not only strengthened the cationic properties of Au but also achieved stronger basic sites after Au loading. Besides, the sHAP support can also adsorb and activate the substrate in the catalytic process. And the Au/sHAPs catalyst performs excellent catalytic activity in the oxidative esterification of octanal with ethanol. The ethyl octanoate yield was 84% at 373 K for 3 h.