1 引言
2 纳米颗粒与超细粉体制备的凝聚态化学
2.1 无机纳米颗粒与超细粉体简述
表1 常见氧化物粉体制备所用化学试剂和合成方法Table 1 Chemical agents and synthetic procedures for the preparation of oxide nanoparticles |
| Polycrystalline ceramicsBoehmite, Ammonia aluminum sulfate, Aluminum organic salts | Chemical agentsPrecipitation + pyrolysis | Synthesis proceduresAlumina (Al2O3) |
|---|---|---|
| Zirconia (ZrO2) | Zirconium oxychloride, Zirconium sulfate, Zirconium organic salts | Precipitation + pyrolysis; Hydrolysis; Hydrothermal |
| Yttria (Y2O3) | Yttrium chloride, Yttrium sulfate, Yttrium nitrate, Yttrium organic salts | Precipitation + pyrolysis; Hydrolysis |
| Barium titanate (BaTiO3) | Barium oxalate, Barium oxide, Titanium oxide, Titanate, Metal organic salts | Solid state reaction, Precipitation + pyrolysis; Hydrolysis |
| Lead zirconium titanate(PZT, PLZT) | Related oxides, carbonates, oxalates, Metal organic salts | Solid state reaction, Precipitation + pyrolysis; Hydrolysis |
| Spinels | Oxides, carbonates, oxalates, Nitrates, Metal organic salts | Solid state reaction, Precipitation + pyrolysis; Hydrolysis |
| Aluminum silicate (Mullite) | Alumina, silica, other inorganic or organic salts | Solid state reaction, Precipitation + pyrolysis; Hydrolysis |
| Silicon Nitrides or other nitrides | Silicon(metal, oxide) powders + nitrogen sources(nitrogen, ammonia) | Solid state nitridation |
| Silicon carbides and other carbides | Silicon(metal, oxide) powders +carbon sources, Si-containing organics | Solid state reaction, Pyrolysis |
2.2 无机纳米颗粒与超细粉体的凝聚态化学特性
2.3 无机纳米颗粒与超细粉体制备的常规化学方法
3 纳米与介孔复合颗粒合成的凝聚态化学
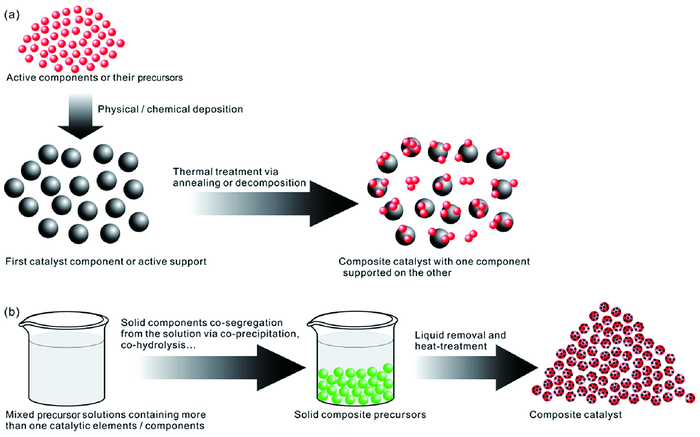
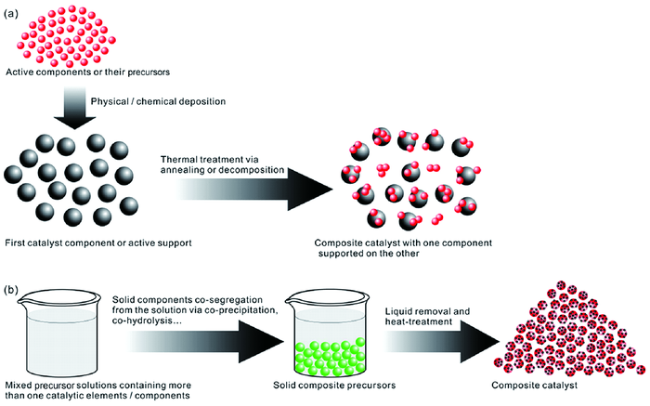
3.1 纳米复合材料制备的凝聚态化学路径描述
3.2 软模板法制备介孔和介孔结构纳米复合材料
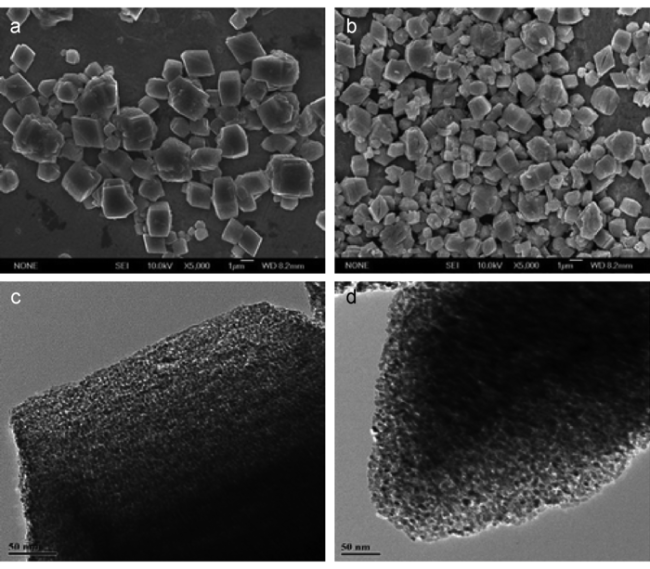
3.3 晶化介孔氧化物和双组分介孔复合材料的反相复制法制备
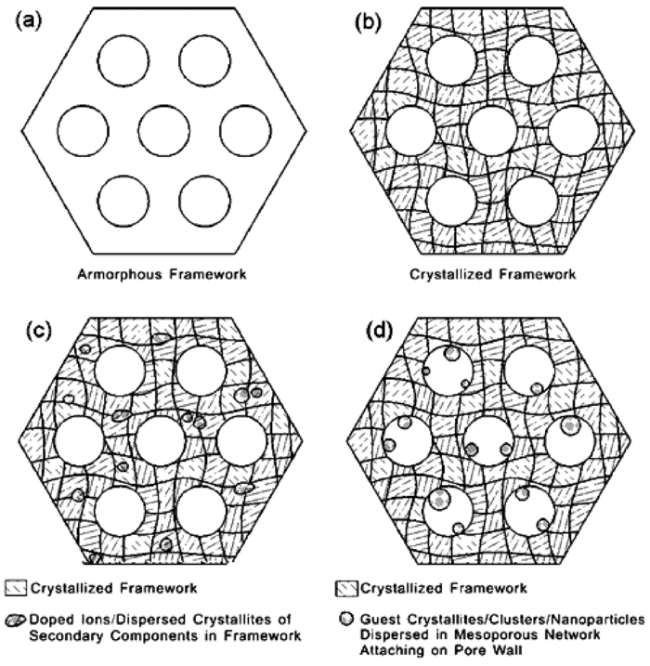
图2 反相复制法制备的介孔结构复合材料骨架结构示意图:(a)非晶骨架;(b)纳米尺度多晶颗粒组成的晶化骨架;(c)客体组分均匀掺杂和/或分散于晶化主体骨架(小圆点代表离子或超小晶粒);以及(d)客体组分(晶粒/团簇/纳米颗粒)负载分散于介孔孔道并贴附于孔道内表面[20]Fig.2 Schematics of mesostructured materials/composites with(a) amorphous framework,(b) crystallized framework composed of numerous nanosized polycrystallites,(c) guest species homogeneously doped or dispersed in the crystallized host framework(small and round dots present the guest ions and/or ultrasmall crystallites), and (d) guests(crystallites/clusters/nanoparticles) loaded/dispersed in the pore channels attaching on the pore surface[20]. Copyright 2013, American Chemical Society |
3.4 无模板法制备介孔金属氧化物和复合材料
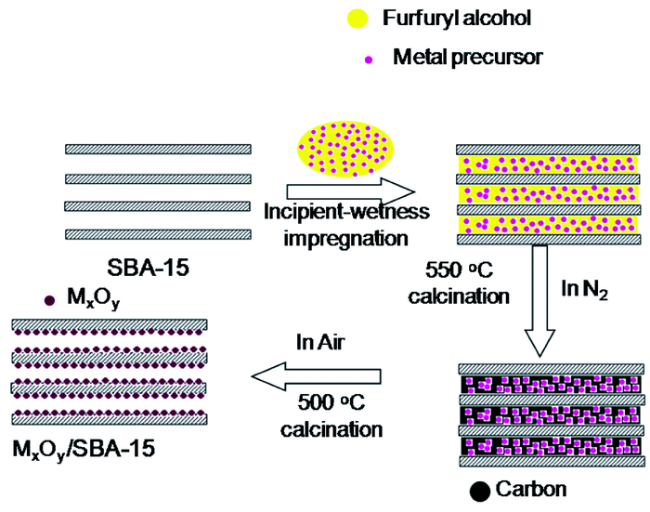
3.5 后负载策略制备介孔主客体复合物
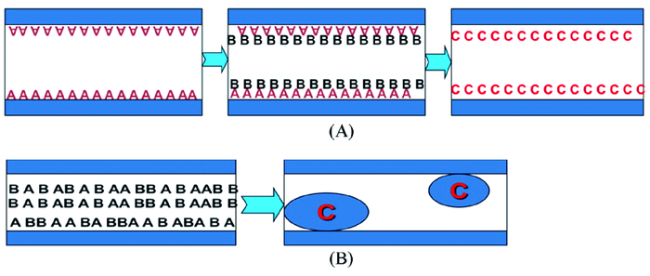
图4 介孔孔道作为化学反应微反应器,在孔道内原位负载客体组分示意图:(A)表面改性法孔道内先引入A,再通过络合/螯合等反应等引入B,得到产物C固定于孔道;(B)孔道内同时引入A和B,通过反应物之间的原位反应形成C [20]Fig.4 Schematics of loading guest species in the mesopore channels of mesoporous hosts by employing the mesopores as the microreactors:(a) Reactant A is first introduced by pore surface modification, followed by the introduction of reactant B via a certain kind of interaction(e.g., complexing);(b) A and B are co-introduced simultaneously into the pore channels, and subsequent in situ reaction between the reactants A and B results in the formation of product C within the pore channels[20]. Copyright 2013, American Chemical Society |