1 引言
2 氧化物气凝胶的制备
2.1 氧化物气凝胶的制备
2.1.1 溶胶-凝胶法
2.1.2 环氧化物添加法
2.1.3 无机溶胶-凝胶法
2.2 干燥方法
2.2.1 超临界干燥
2.2.2 常压干燥
2.2.3 冷冻干燥
2.2.4 微波干燥
3 SiO2气凝胶
表1 SiO2气凝胶的制备方法与性能a)Table 1 Preparation method and properties of SiO2 aerogela) |
| Precursor | Drying method | Modified way | Service temperature /℃ | Specific surface area/ (m2/g) | Thermal conductivity/ (W/(m·K)) | Ref. |
|---|---|---|---|---|---|---|
| TMOS | Supercritical drying | − | − | − | 0.0135 (RT) | 44 |
| Silica sol | Hydrothermal | 1200 | 156 (RT) 148 (1200 ℃) | − | 48 | |
| MTMS | Insert flexible ether group | − | − | 0.0159 (RT) | 50 | |
| TEOS | Add ZrCl4/AlCl3 | 1200 | 653.67 (RT ZrCl4) 524.32 (RT AlCl3) | − | 53 | |
| P-VTMS | Add TPU | − | 2145 (RT) | 0.026 (RT) | 54 | |
| TEOS | Doped with Y2O3 | 900 | 1010.4 (RT) 643.8 (900 ℃) | − | 56 | |
| Silica sol | Atmospheric pressure drying | − | 700 | 333.43 (RT) | 0.019 (RT) 0.044 (600 ℃) | 46 |
| Nano SiO2 aqueous liquid slurry | Add fiberglass felt | − | − | 0.02846 (100 ℃) 0.05457 (300 ℃) 0.09367 (500 ℃) 0.1506 (700 ℃) | 47 | |
| TMOS | Age | 500 | 595 (RT) | − | 25 | |
| TEOS、HMDZ | Solvent displacement | − | 750 (RT) | 0.07 (RT) | 26 | |
| TEOS | Solvent displacement | − | 530 (RT) | − | 27 | |
| TEOS | Hydrophobic modification | − | 973 (RT HMDS) 1067 (RT TMCS) | − | 29 | |
| TEOS、MTMS | − | 300 | 364.5 (RT) 895.5 (300 ℃) | − | 30 | |
| TEOS | Vapor deposition BN | 700 | 526 (RT) 252 (RT BN) | 0.083 (RT) 0.090 (BN) | 31 | |
| TEOS | Adding SiO2 nanofibers and hydrophobic modification | − | 624.19 (RT) | 0.021 (RT) | 52 | |
| Nano SiO2 aqueous liquid slurry | Add fiberglass felt and SiC | − | − | 0.1334 (700 ℃) | 47 | |
| TEOS | Doped with Y2O3 | 727 | 917.5 (RT) | 0.051 (RT) 0.080(727 ℃) | 55 | |
| TEOS | Freeze-drying | Electrospinning | 1100 | 0.024 (RT) 0.036 (300 ℃) | 44 |
a) TEOS:Ethyl orthosilicate;TMOS:Methyl silicate;MTMS:Methyltrimethoxysilane;P-VTMS:Polyethylene based trimethoxysilane;TPU:Thermoplastic Polyurethane; HMDS:Hexamethyldisilazane;TMCS:Trimethylsilyl chloride;RT:Room Temperature |
3.1 SiO2气凝胶的前驱体
3.1.1 有机前驱体
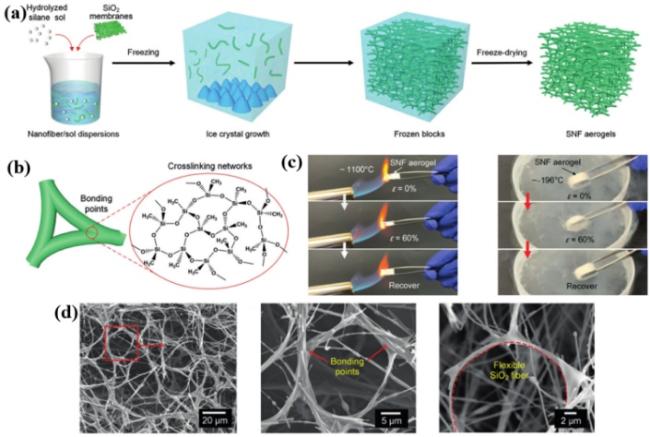
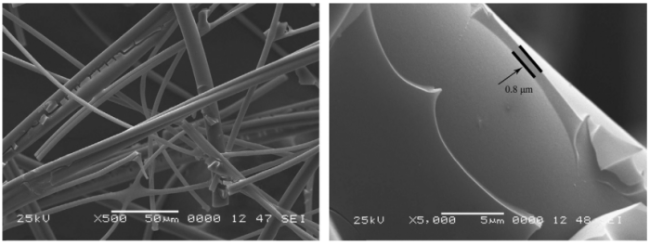
图2 (a) SiO2纳米纤维气凝胶的制造的示意图;(b) SiO2纳米纤维气凝胶中纳米纤维之间的交联网络;(c) SiO2纳米纤维气凝胶在丁烷喷灯(1100 ℃)和液氮(−196 ℃)的火焰中;(d) SiO2纳米纤维气凝胶的微观结构[44]Fig. 2 (a) Schematic illustration showing the fabrication of SiO2 nanofiber aerogel. (b) The crosslinking networks between the nanofibers of SiO2 nanofiber aerogel. (c) SNF aerogels in flame of a butane blowtorch (1100 ℃) and liquid nitrogen (−196 ℃). (d) Microscopic architecture of an SiO2 nanofiber aerogel[44] |
3.1.2 无机前驱体
3.2 SiO2气凝胶的预处理
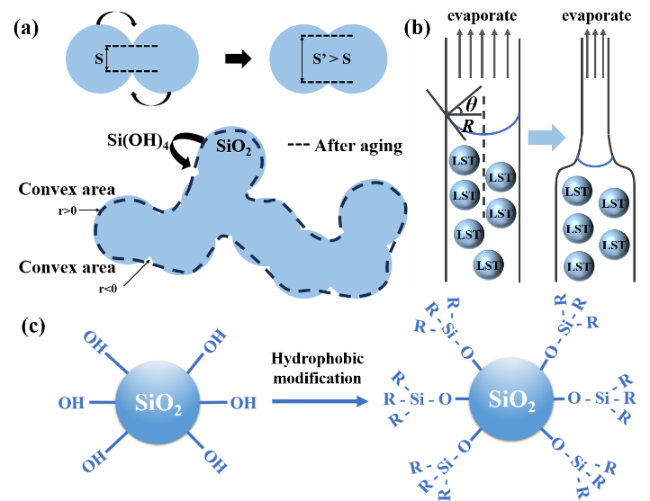
3.2.1 老化处理
3.2.2 低表面能溶剂置换
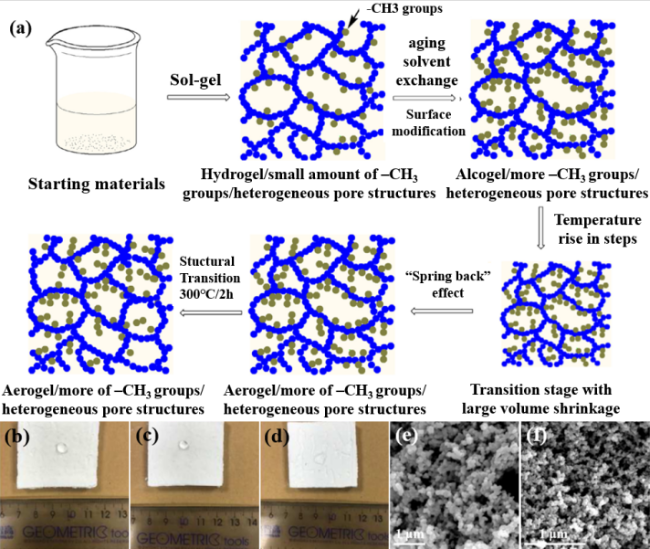
3.2.3 疏水改性
图4 (a) 常压干燥法制备柔性疏水二氧化硅气凝胶的示意图;将水滴置于干燥后的疏水二氧化硅气凝胶(b)与在300 ℃ (c)和400 ℃ (d)热处理后的疏水二氧化硅气凝胶光学图片;以MTMS和TEOS为共前驱体(MTMS体积百分比为60%),经TMCS表面改性后凝胶的SEM图像(e)和300 ℃热处理后(f)二氧化硅气凝胶的SEM图像及结构[30]Fig. 4 (a) Schematic diagram of forming flexible hydrophobic silica aerogel by APD process. Place the water drop on the dried hydrophobic silica aerogel (b) and the optical picture of hydrophobic silica aerogel after heat treatment at 300 ℃ (c) and 400 ℃ (d). SEM images of gel after surface modification of TMCS with MTMS and TEOS as co precursors (MTMS volume percentage is 60%) (e) and 300 ℃ heat treatment (f)[30] |
3.2.4 聚合物增强
3.3 SiO2复合气凝胶
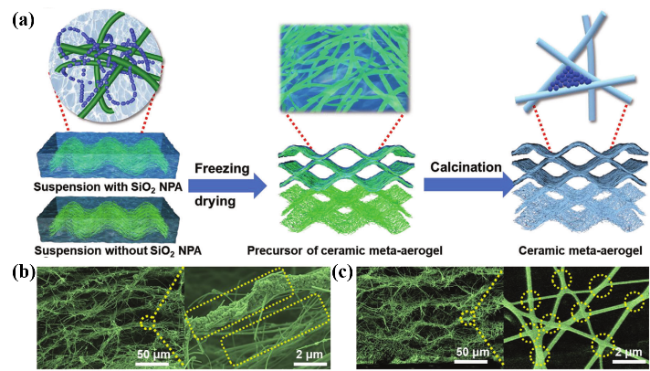
3.3.1 纤维增强SiO2复合气凝胶
3.3.2 加入遮光剂的SiO2复合气凝胶
4 Al2O3气凝胶
表2 Al2O3气凝胶的制备方法与性能a)Table 2 Preparation method and properties of Al2O3 aerogela) |
| Precursor | Drying method | Modified way | Service temperature /℃ | Specific surface area/ (m2/g) | Thermal conductivity/ (W/(m·K)) | Ref. |
|---|---|---|---|---|---|---|
| ASB | Supercritical drying | Add ceramic fiber felt | 1000 | - | 0.022 (RT) 0.058 (600 ℃) 0.092 (1000 ℃) | 58 |
| AIP | - | - | 376 (RT) | 0.029 (RT) | 59 | |
| ASB | ISWF Method Combined with SCFM and HMDS Gas Phase Modification | 1300 | 152~261 (1200 ℃) 125~136 (1300 ℃) | 0.05 (RT) | 60 | |
| AlCl3·6H2O | Doped with Si | 1200 | 283 (1000 ℃) | 0.035 (RT) | 75 | |
| AlCl3·6H2O | Doped with Si | 1200 | 515 (RT) 217 (1000 ℃) 68 (1200 ℃) | 0.025 (150 ℃) 0.121 (1200 ℃) | 76 | |
| AIP | Carbon coated Al2O3 nanorods | 1400 | - | 0037 (RT) 0.065 (1200 ℃) | 77 | |
| AlCl3·6H2O | Doped with SrO | 1200 | 122 (1200 ℃) | 0.060 (RT) | 80 | |
| AlCl3·6H2O | Doped with Y2O3 | 1000 | 380~400 (1000 ℃) | - | 81 | |
| Al(NO3)3·9H2O | Introducing SiO2 fiber felt | 900 | - | 0.028 (35 ℃) 0.033 (600 ℃) | 83 | |
| AlCl3·6H2O | Adding mullite fibers | 1400 | - | 0.058 (200 ℃) 0.152 (1400 ℃) | 84 | |
| AlCl3·6H2O | Doped with TiO2 | 1000 | 650 (RT) | 0.136 (1000 ℃) | 86 | |
| AlCl3·6H2O | Atmospheric pressure drying | - | 1000 | 465 (RT) | - | 60 |
| Al(NO3)3·9H2O | Freeze-drying | Preparation α- Al2O3 nanosheets bonded with silica sol | 1600 | - | 0.029 (RT) | 65 |
| AlCl3·6H2O | Using chitosan as a template and using solution freezing drying calcination technology | 1300 | 250 (RT) | - | 69 | |
| Al2O3 nanorod sol | PVA bonding and doped with Si | 1400 | 118 (RT) 39.12 (1400 ℃) | 0.0246 (RT) 0.0949 (1000 ℃) | 78 | |
| ASB | Freeze-drying | Adding TEOS and SiO2 aerogel nanoparticles, Introducing inert inorganic molecular chains | 1700 | - | 0.028 (RT) | 79 |
| ASB | - | Combining Blow Spinning and Atomic Layer Deposition (ALD) | 900 | - | 0.022 (RT) | 68 |
a) ASB:Aluminum sec-butoxide;AIP:Aluminum isopropoxide;ISWF:Acetone Aniline in situ Water Formation;SCFM:Supercritical Fluid Modification;HMDS:Hexamethyldisilazane;TEOS:Ethyl orthosilicate;RT:Room Temperature |
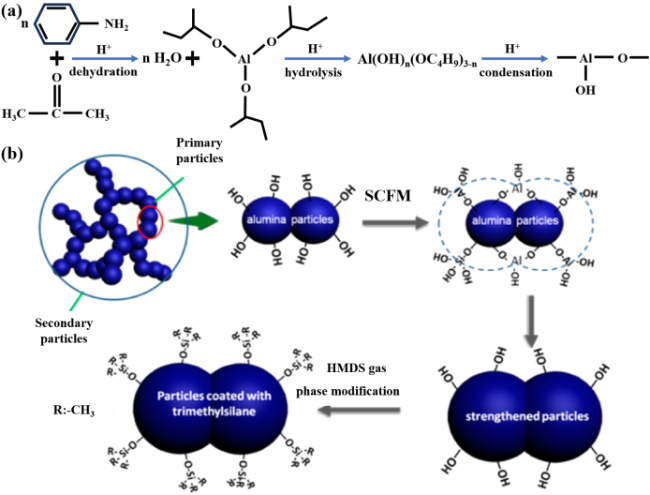
4.1 Al2O3气凝胶的前驱体
4.1.1 有机前驱体
4.1.2 无机前驱体
4.2 Al2O3气凝胶的结构调控
4.3 Al2O3复合气凝胶
 γ
γ 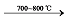 δ
δ 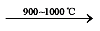 θ
θ 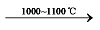 α-Al2O3的相变[70]。γ-AlOOH脱羟基后,Al2O3晶格中的活化原子在高温下会迁移扩散,O2-由立方向六角密堆积转化,而随机分布于八面体或四面体空隙中的Al3+则会均匀分布在八面体空隙中,最终相变形成稳定的α-Al2O3[71]。AlOOH、γ-Al2O3和δ-Al2O3都为尖晶石结构,而α-Al2O3为密排六方结构,所以在1000 ℃以上发生α相变会使体积收缩,结构被破坏,导致其无法在高温条件下使用。Al2O3气凝胶在高温下也会使Al2O3颗粒之间发生烧结[72],导致Al2O3表面能的降低和颗粒聚集长大,Al2O3颗粒比表面积降低。因此,提高Al2O3热稳定性的研究主要集中于抑制α相变和高温烧结。
α-Al2O3的相变[70]。γ-AlOOH脱羟基后,Al2O3晶格中的活化原子在高温下会迁移扩散,O2-由立方向六角密堆积转化,而随机分布于八面体或四面体空隙中的Al3+则会均匀分布在八面体空隙中,最终相变形成稳定的α-Al2O3[71]。AlOOH、γ-Al2O3和δ-Al2O3都为尖晶石结构,而α-Al2O3为密排六方结构,所以在1000 ℃以上发生α相变会使体积收缩,结构被破坏,导致其无法在高温条件下使用。Al2O3气凝胶在高温下也会使Al2O3颗粒之间发生烧结[72],导致Al2O3表面能的降低和颗粒聚集长大,Al2O3颗粒比表面积降低。因此,提高Al2O3热稳定性的研究主要集中于抑制α相变和高温烧结。4.3.1 掺杂元素的Al2O3复合气凝胶
图6 (a) Si掺杂Al2O3纳米棒气凝胶制备示意图;(b) Si掺杂Al2O3纳米棒气凝胶在干燥后和在不同温度下处理后的照片;(c) Si掺杂Al2O3纳米棒气凝胶在1400 ℃下的SEM图像[78]Fig. 6 (a) Schematic diagram of preparation of Si doped Al2O3 nanorod aerogels; (b) Macroscopic photographs of Si doped Al2O3 nanorod aerogel after drying and after treatment at different temperatures; (c) SEM image of Si doped Al2O3 nanorod aerogel at 1400 ℃ [78] |
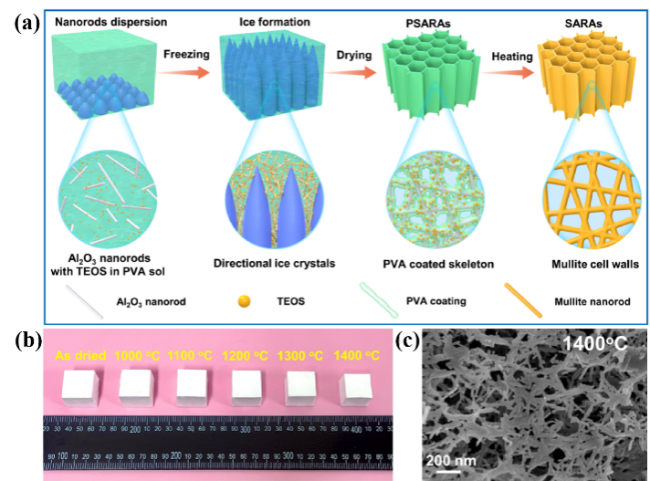
4.3.2 纤维增强Al2O3气凝胶
4.3.3 加入遮光剂的Al2O3气凝胶
图9 (a) RF包覆Al2O3纳米棒气凝胶、碳包覆Al2O3纳米棒气凝胶和Al2O3纳米棒气凝胶制备示意图;(b) Al2O3纳米棒和RF层之间的强耦合界面示意图;(c) RF包覆Al2O3纳米棒气凝胶,(d)碳包覆Al2O3纳米棒气凝胶和 (e) Al2O3纳米棒气凝胶的SEM图像[77]Fig. 9 (a) Preparation schematic diagram of RF coated Al2O3 nanorod aerogel, carbon coated Al2O3 nanorod aerogel and Al2O3 nanorod aerogel. (b) Schematic illustration of the strong interfacial coupling between Al2O3 nanorods and RF layers. (c) SEM images of RF coated Al2O3 nanorod aerogels, (d) carbon coated Al2O3 nanorod aerogels and (e) Al2O3 nanorod aerogels[77] |
5 ZrO2气凝胶
表3 ZrO2气凝胶的制备方法与性能a)Table 3 Preparation method and properties of ZrO2 aerogela) |
| Precursor | Drying method | Modified way | Service temperature /℃ | Specific surface area/(m2/g) | Thermal conductivity/ (W/(m·K)) | Ref. |
|---|---|---|---|---|---|---|
| ZBO | Supercritical drying | - | 500 | 178 (RT) | - | 90 |
| (C5H8O2)4·Zr | Doped with SiO2 | 1000 | - | 0.026 (600 ℃) 0.037 (800 ℃) 0.058 (1000 ℃) | 91 | |
| ZrO(NO₃)₂ | Introducing formamide | 800 | 514.5 (RT) | - | 93 | |
| ZrOCl2·8H2O | Electrolytic method | - | 640 (RT) | - | 94 | |
| ZrO(NO₃)₂ | - | 1000 | 223 (1000 ℃) | - | 96 | |
| ZrO(NO₃)₂ | Alcohol water heating method | 1000 | 675.6 (RT) | - | 97 | |
| ZrOCl2 | Doped with La | 1200 | 107(1000 ℃) | 106 | ||
| ZrO(NO₃)₂ | Atmospheric pressure drying | - | - | 645 (RT) | - | 98 |
| ZrO(NO3)2 | Add formamide and heat with alcohol and water | - | 619 (RT) | - | 99 | |
| ZrO(NO₃)₂ | Freeze-drying | Gel casting process | 900 | - | - | 101 |
a) ZBO:N-butanol zirconium;RT:Room Temperature |
5.1 ZrO2气凝胶前驱体
5.1.1 有机前驱体
5.1.2 无机前驱体
5.2 ZrO2气凝胶的结构调控
5.3 复合ZrO2气凝胶
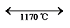 t-ZrO2
t-ZrO2 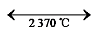 c-ZrO2
c-ZrO2 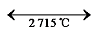 液相-ZrO2的相变[102]。四方相ZrO2具有热导率低、机械强度高等优点。单斜相ZrO2向四方相ZrO2转变的过程中会产生压应力和拉应力,无法被基体的弹性变形和塑性变形抵消,导致ZrO2气凝胶开裂,严重限制了ZrO2气凝胶的应用。
液相-ZrO2的相变[102]。四方相ZrO2具有热导率低、机械强度高等优点。单斜相ZrO2向四方相ZrO2转变的过程中会产生压应力和拉应力,无法被基体的弹性变形和塑性变形抵消,导致ZrO2气凝胶开裂,严重限制了ZrO2气凝胶的应用。6 二元和多元氧化物气凝胶
表4 二元和多元氧化物气凝胶的制备方法与性能a)Table 4 Preparation method and properties of two component and multi-component oxide aerogela) |
| Precursor | Drying method | Modified way | Service temperature/℃ | Specific surface area/ (m2/g) | Thermal conductivity/ (W/(m·K)) | Ref. |
|---|---|---|---|---|---|---|
| ASB、TEOS | Supercritical drying | - | 1200 | 97~116 (1200 ℃) | - | 108 |
| Water glass、AlCl3 | Al:Si=0.37 | 1200 | 613 (RT) 11.8 (1000 ℃) | 0.029 (RT) 0.121 (1200 ℃) | 109 | |
| ASB、TEOS | Mullite fibers impregnated with SiC coating | 1000 | - | 0.049 (1000 ℃) | 110 | |
| AlCl3·6H2O、TEOS | Impregnated ZrO2 fibers | 800 | - | 0.049 (RT) | 111 | |
| TEOS、Al(NO3)3·9H2O | Aluminum silicate fiber reinforcement | 1200 | 600 (RT) 40 (1300 ℃) | 0.026 (RT) | 112 | |
| ZrOCl2·8H2O、TEOS | Supercritical fluid deposition | 1000 | 551 (RT) 50 (1300 ℃) | - | 113 | |
| ZrOCl2、TEOS | ZrO2 fiber reinforcement | - | - | 0.0235~0.0296 (RT) | 114 | |
| ZrOCl2、TEOS | Impregnated mullite fiber | 1200 | - | 0.0524 (RT) | 115 | |
| ZrOCl2·8H2O、TEOS | Multiple impregnation of ZrO2-SiO2 sol | - | - | 0.0231~0.0306 (RT) | 116 | |
| TEOS、AlCl3·6H2O、ZrOCl2·8H2O | - | 800 | - | 0.05 (RT) 0.26 (1000 ℃) | 117 | |
| TEOS、AlCl3·6H2O、MgCl2·6H2O | - | 800 | - | 0.06 (RT) 0.3 (1000 ℃) | 119 | |
| TEOS、AlCl3·6H2O、ZrOCl2·8H2O、MgCl2·6H2O | - | 1200 | 597.22 (RT) 358.53 (1000 ℃) 84.44 (1200 ℃) | 0.03 (RT) | 119 | |
| AlCl3·6H2O、AIP、C8H12O8·Zr | Freeze-drying | Gel casting process | 1300 | - | 0.1602~0.1623 (RT) | 118 |
| AlCl3·6H2O、AIP、Zr(CO3)2 | - | electrospinning | 1300 | - | 0.03166 (RT) | 117 |
a) ZBO:N-butanol zirconium;AIP:Aluminum isopropoxide;TEOS:Ethyl orthosilicate;RT:Room Temperature |
6.1 二元氧化物气凝胶
6.1.1 Al2O3-SiO2气凝胶
6.1.2 ZrO2-SiO2气凝胶
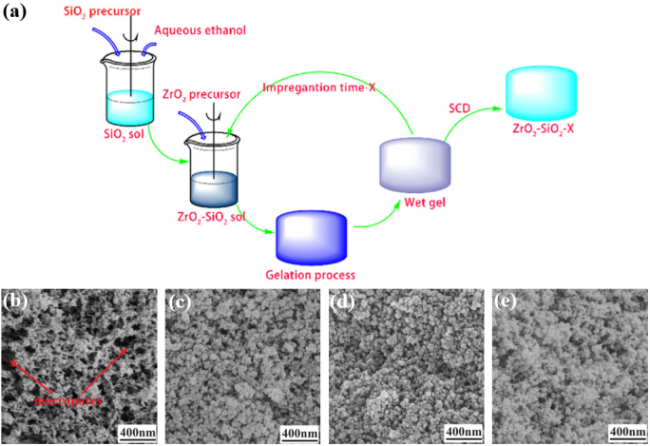
图11 (a) 浸渍法制备ZrO2-SiO2气凝胶工艺流程图;不同浸渍次数的0次(b),1次(c),2次(d),3次(e)自增强ZrO2-SiO2气凝胶SEM图像[116]Fig. 11 (a) Flow chart of the preparation processing for ZrO2-SiO2 aerogel fabricated by impregnation method.SEM of the nanostructure self-reinforcing ZrO2-SiO2 aerogels with different impregnation times. (b) ZrO2-SiO2-0; (c): ZrO2-SiO2-1; (d): ZrO2-SiO2-2; (e): ZrO2-SiO2-3[116] |