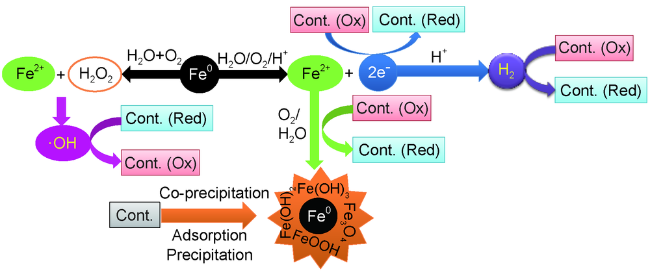
Contents
1 引言
2 碳材料稳定化纳米零价铁的制备方法
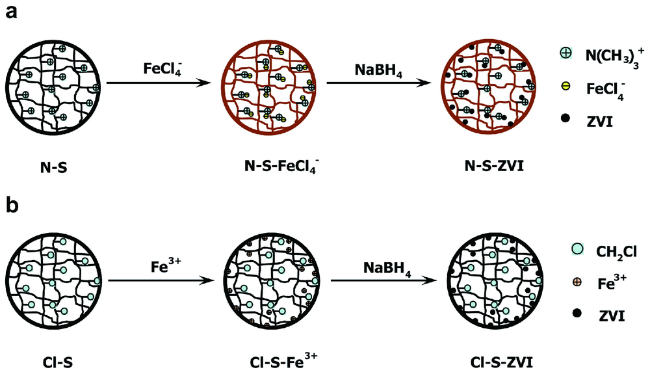
2.1 包覆型nZVI
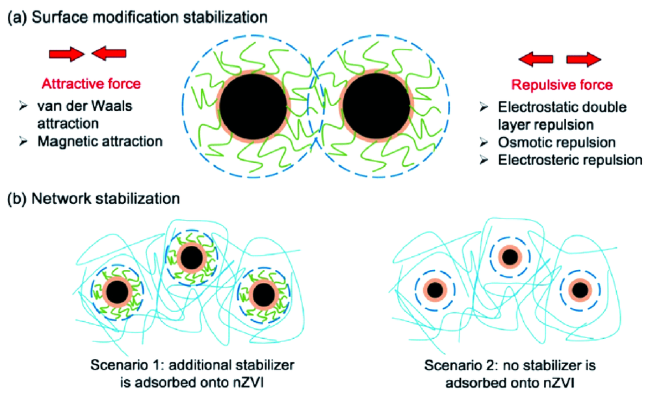
图2 包覆型纳米零价铁的制备及稳定机理示意图(a)改变表面电荷以引起颗粒互斥,(b)构建网格结构以提供空间位阻[30]Fig. 2 Schematic representation of(a) surface modification stabilization(where surface coating facilitates particle repulsion), and(b) network stabilization(where a medium network is formed due to hydrogen bonding and polymer entanglements)[30] |
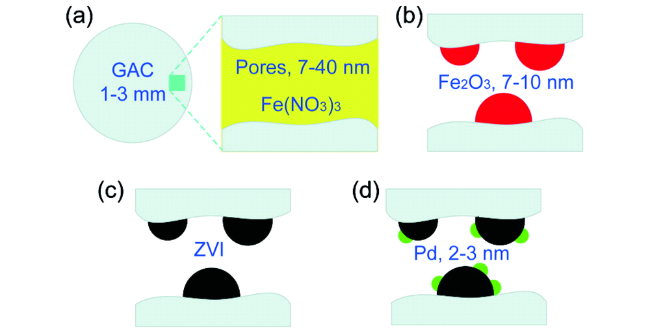
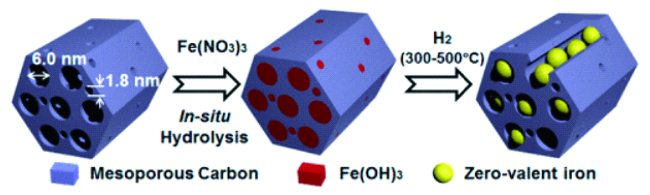
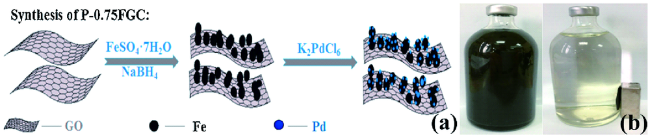
2.2 负载型nZVI
3 碳材料修饰对纳米零价铁迁移性、反应活性及选择性的影响
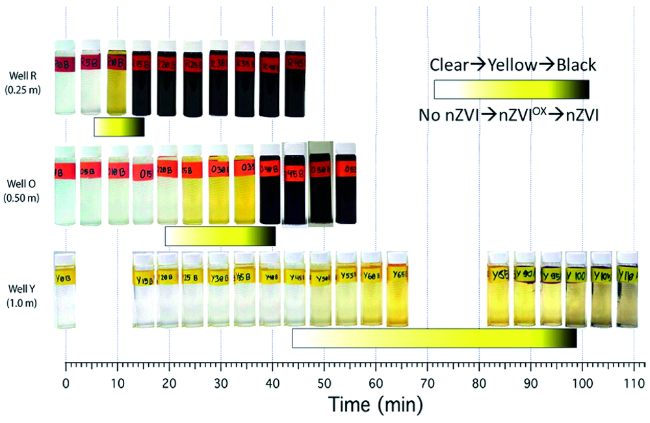
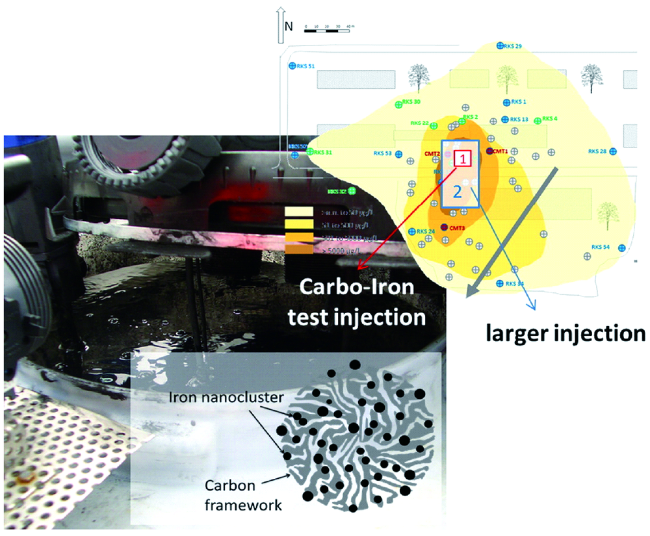
3.1 碳材料修饰对nZVI迁移性的影响
图7 CMC/nZVI在不同距离监测井处随时间的迁移分布情况[50]。黄色、黑色分别代表被氧化后的CMC/nZVIox及未反应的CMC/nZVI,颜色的深浅示意浓度的高低Fig. 7 Composite photograph of water samples taken from the first three sample wells over the time period of the injection test. Rectangular markers highlight the location of the two color transitions that indicate breakthrough of CMC/nZVIox(yellow) and CMC/nZVI(black)[50] |
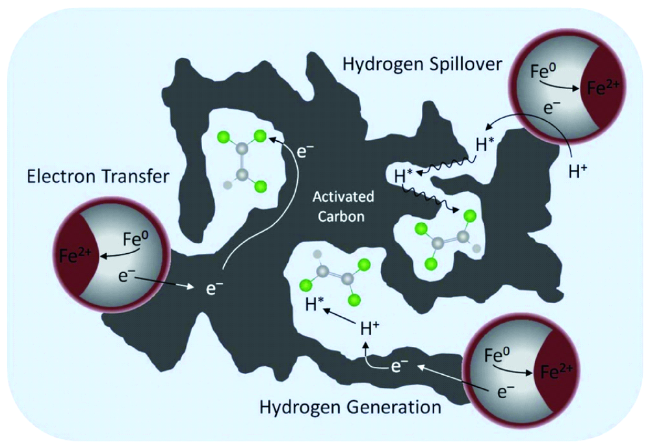
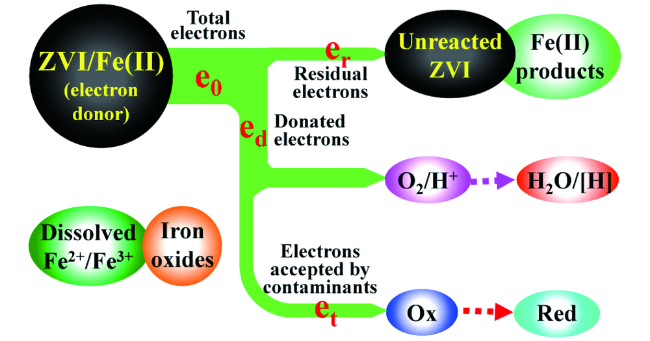
3.2 碳材料修饰对nZVI反应活性的影响
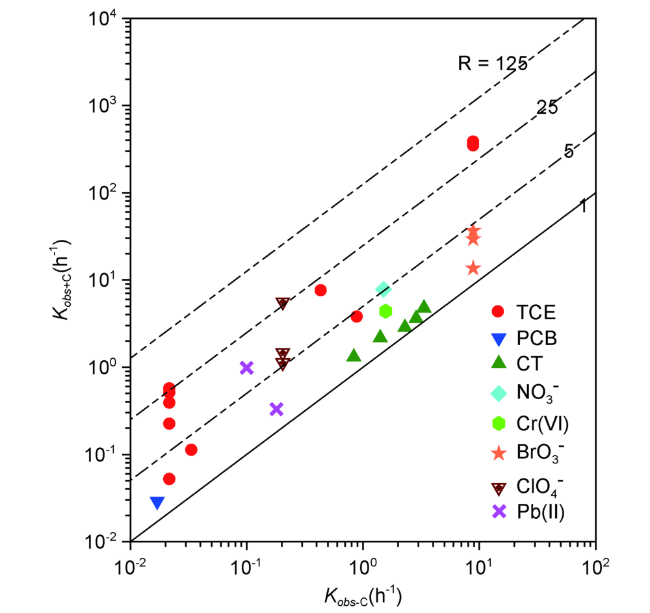
表1 碳材料修饰对nZVI去除不同污染物速率常数的影响Table 1 Summary of data on the removal rate of various contaminants by bare nZVI and carbon-modified nZVI |
| nZVI type | Cont. | Reaction conditons | (h-1) | (h-1) | ref | |
|---|---|---|---|---|---|---|
| Coated nZVI | CMC | TCE | TCE=50 mg/L, Fe0=0.1 g/L,0.1%Pd, CMC90 K | 0.381 | 0.022 | 54 |
| TCE=50 mg/L, Fe0=0.1 g/L,0.1%Pd, CMC250 K | 0.557 | 0.022 | ||||
| TCE=50 mg/L, Fe0=0.1 g/L,0.1%Pd, CMC700 K | 0.497 | 0.022 | ||||
| PVP | TCE=50 mg/L, Fe0=0.1 g/L,0.1%Pd, PVP360 K | 0.219 | 0.022 | |||
| GG | TCE=50 mg/L, Fe0=0.1 g/L,0.1%Pd, GG 0.05% | 0.051 | 0.022 | |||
| CMC | TCE | TCE=50 mg/L, Fe0=0.1 g/L | 7.4 | 0.44 | 27 | |
| Starch | TCE | TCE=25 mg/L, Fe0=0.1 g/L | 0.11 | 0.034 | 55 | |
| TCE=25 mg/L, Fe0=0.1 g/L, 0.1%Pd | 3.7 | 0.9 | ||||
| PCB | PCB=2.5 mg/L, Fe0=0.1 g/L, 0.1%Pd | 0.029 | 0.017 | |||
| CMC | Pb(Ⅱ) | Pb(Ⅱ)=200 mg/L, Fe0=0.75 g/L, pH=5.0 | 1.12 | 0.204 | 56 | |
| Starch | Pb(Ⅱ)=200 mg/L, Fe0=0.75 g/L, pH=5.0 | 1.46 | 0.204 | |||
| Agar | Pb(Ⅱ)=200 mg/L, Fe0=0.75 g/L, pH=5.0 | 5.60 | 0.204 | |||
| CMC | NO3- | NO3-=200 mg/L, Fe0=0.7 g/L, pH=~7.0 | 7.8 | 1.5 | 57 | |
| CMC | ClO4- | ClO4-=10 mg/L, Fe0=1.8 g/L, pH=~7.0, 110 ℃ | 0.33 | 0.18 | 58 | |
| Starch | ClO4-=10 mg/L, Fe0=1.8 g/L, pH=~7.0, 110 ℃ | 0.984 | 0.1 | |||
| Supported nZVI | Graphene oxide | CT | CT=3 mg/L, Fe0=0.5 g/L, pH=5.5, T=10 ℃ | 1.308 | 0.834 | 46 |
| CT=3 mg/L, Fe0=0.5 g/L, pH=5.5, T=20 ℃ | 2.166 | 1.404 | ||||
| CT=3 mg/L, Fe0=0.5 g/L, pH=5.5, T=30 ℃ | 2.844 | 2.292 | ||||
| CT=3 mg/L, Fe0=0.5 g/L, pH=5.5, T=35 ℃ | 3.618 | 2.862 | ||||
| CT=3 mg/L, Fe0=0.5 g/L, pH=5.5, T=40 ℃ | 4.776 | 3.342 | ||||
| GAC | TCE | TCE=80 mg/L, Fe0=0.15 g/L, GAC-105 ℃ | 339.6 | 9 | 36 | |
| TCE=80 mg/L, Fe0=0.15 g/L, GAC-700 ℃ | 374.4 | 9 | ||||
| AC | BrO3- | BrO3-=0.2 mg/L, Fe0=5 g/L, pH=~7.0 | 13.62 | 8.84 | 59 | |
| BrO3-=0.2 mg/L, Fe0=5 g/L, pH=~7.0 | 29.4 | 8.84 | ||||
| BrO3-=0.2 mg/L, Fe0=5 g/L, pH=~7.0 | 36.7 | 8.84 | ||||
| Graphene oxide | Cr(Ⅵ) | Cr(Ⅵ)=25 mg/L, Fe0=1.0 mg/L | 4.38 | 1.56 | 60 |
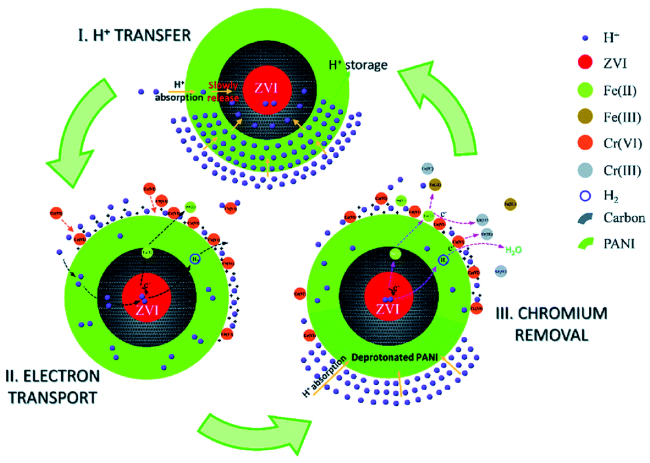
3.3 碳材料修饰对nZVI选择性的影响
4 结论与展望
表2 典型碳材料修饰nZVI效果的比较Table 2 Summary of the effects of typical carbon materials on modifying nZVI |
| Carbon-based materials | Key properties | Remarks | ref | |
|---|---|---|---|---|
| Surfactants | SDBS | Anionic surfactants electrostatic repulsion | Relative weak stabilization effect; limited transportability in real soil; the introduced surfactants in the subsurface may solubilize/mobilize non-targeted contaminants | 33 |
| Tween 20 | Nonionic surfactant Network stabilization | 34 | ||
| Synthetic polymers | CMC | Food grade polysaccharide. Nontoxic and biodegradable. Weak anionic functional groups(pKa=4.3). MW=90 or 700 kDa. CMC binds with nZVI through bidentate bridging. | Very effective stabilization effect; better transportability than surfactant-coated nZVI; no enhancing effect on nZVI selectivity | 23, 35, 40, 49, 50 |
| PVP | Neutral polyelectrolyte. MW=40 or 360 kDa. | Less effective than CMC; may enhance transportability of nZVI; no enhancing effect on nZVI selectivity | 54 | |
| Natural biopolymers | Starch | Neutral polysaccharide. nZVI-starch interactions and formation of intrastarch Fe clusters play a fundamental role in stabilizing nZVI. | Effective stabilization effect; may enhance transportability of nZVI; no enhancing effect on nZVI selectivity | 55, 58 |
| Guar gum | Neutral polysaccharide. It binds with nZVI via the hydroxyl groups. | More effective stabilization effect than starch; may enhance transportability of nZVI; no enhancing effect on nZVI selectivity | 54, 56 | |
| Solid supports | AC | Highly porous internal structure; ideal adsorption property. | Ideal supports or vehicles for stabilizing and delivering nZVI into porous media; Carbo-Iron® could highly enhance ZVI selectivity; inexpensive | 25, 39 |
| Mesoporous carbon | Ordered mesoporous carbon, high surface area, good electroconductivity. | Good supports for stabilizing and delivering nZVI into porous media; may enhance ZVI selectivity; expensive | 10, 43 | |
| Graphene oxide | Consisting of a single layer of carbon atoms arranged in a hexagonal lattice. | Good supports for stabilizing and delivering nZVI into porous media; could enhance ZVI reactivity and selectivity; expensive | 45, 46 | |