 PDF(8164 KB)
PDF(8164 KB)


 PDF(8164 KB)
PDF(8164 KB)
 PDF(8164 KB)
PDF(8164 KB)
光电催化分解水Ⅲ-Ⅴ族半导体光电极薄膜保护策略
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Thin Film Protection Strategy of Ⅲ-Ⅴ Semiconductor Photoelectrode for Water Splitting
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}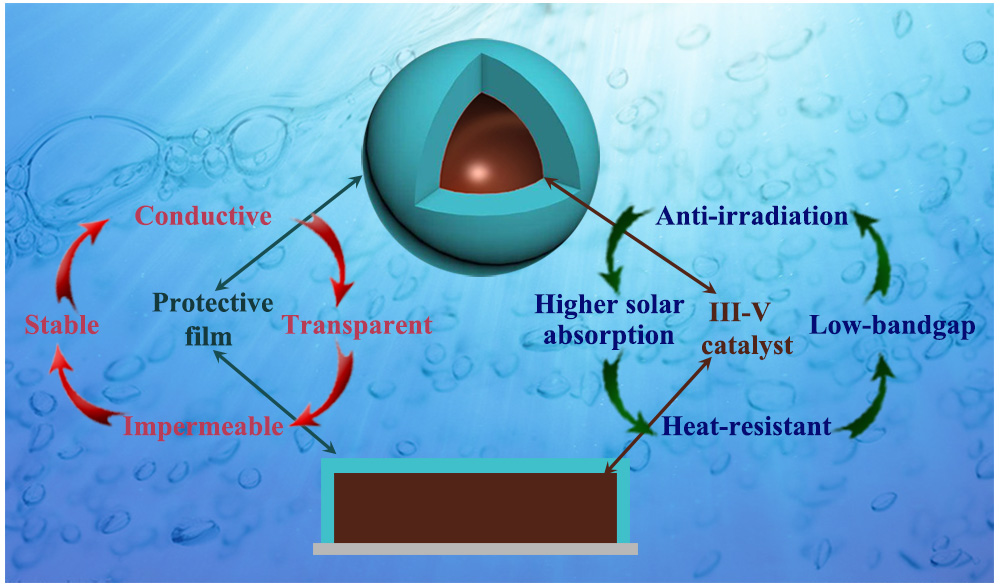
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |