 PDF(14065 KB)
PDF(14065 KB)


 PDF(14065 KB)
PDF(14065 KB)
 PDF(14065 KB)
PDF(14065 KB)
金属配合物用于细胞内动态实时荧光示踪研究
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Real-Time Luminescence Tracking in Living Cells with Metal Complexes
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}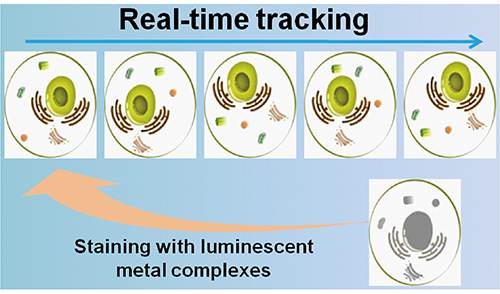
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |