 PDF(2803 KB)
PDF(2803 KB)


 PDF(2803 KB)
PDF(2803 KB)
 PDF(2803 KB)
PDF(2803 KB)
自支撑型过渡金属磷化物电催化析氢反应研究
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Self-Supporting Transition Metal Phosphides as Electrocatalysts for Hydrogen Evolution Reaction
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}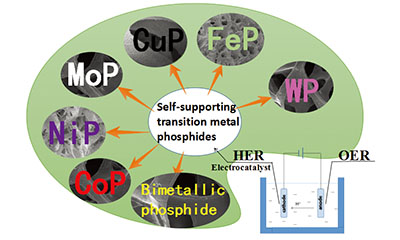
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |