 PDF(7791 KB)
PDF(7791 KB)


 PDF(7791 KB)
PDF(7791 KB)
 PDF(7791 KB)
PDF(7791 KB)
局域表面等离子体共振效应在光催化技术中的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Applications of Localized Surface Plasmon Resonance Effect in Photocatalysis
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}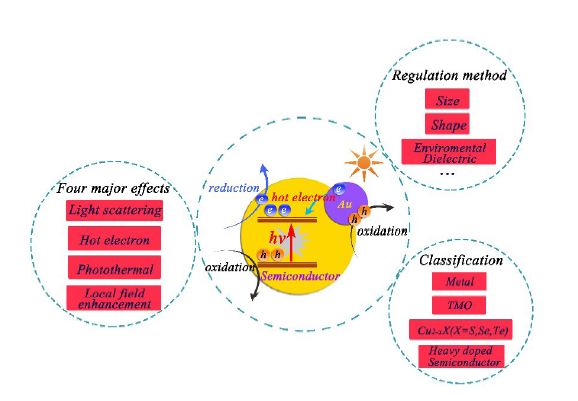
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |