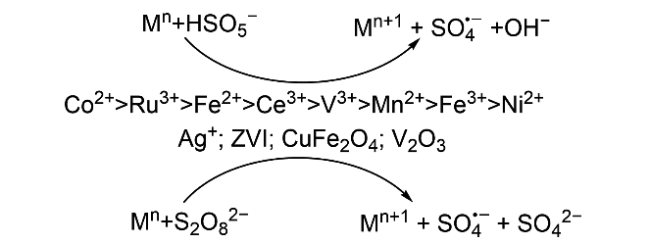1 引言
2 PS的活化方法
2.1 热活化法
2.2 机械化学活化法
2.3 过渡金属活化法
表1 过渡金属活化PS降解有机污染物Table 1 Degradation of organic pollutants by transition metal activated PS |
| Class | Pollutant | Conc. (μM) | PS | Conc. (mM) | Activator | Conc. | pH | T(℃) | t(h) | kobs () | Degradation rate | ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| POPs | BDE-47 | 3.1 | PDS | 71.4 | 23,032∶1 | nZVI | 1000 mg/L | 6~11 | 25 | 48 | — | 64% | 39 |
| BDE-209 | 10.4 | PDS | 200 | 19,183∶1 | Fe2+ | 100 mM | 3~7 | — | 6 | 0.48 | 66% | 41 | |
| CB-28 | 3.9 | PDS | 2 | 500∶1 | V4+ | 1 mM | 5.5 | 25 | 25 | 0.03 | 80% | 34 | |
| phenanthrenea | 5.6 | PDS | 2 | 357∶1 | V2O3 | 100 mg/L | 3~5 | 25 | 3.6 | 7.15 | 88% | 42 | |
| PPCPs | carbamazepine | 21.2 | PMS | 1.66 | 78∶1 | Co3MnFeO6 | 200 mg/L | 3~10 | 35 | 0.5 | 10.98 | 100% | 36 |
| tetracycline | 225 | PDS | 5 | 22∶1 | Ag/AgCl/(0.05)FeX | 1000 mg/L | 3.5 | 25 | 2 | — | 100% | 43 | |
| norfloxacin | 15 | PMS | 1 | 67∶1 | Co0.4Fe2.6O4 | 200 mg/L | 6~8.5 | 40 | 0.05 | 122.40 | ~100% | 44 | |
| norfloxacin | 31.3 | PMS | 2 | 64∶1 | Co(Ⅱ)/TiO2 | 500 mg/L | 3~9 | 25 | 1 | 29.88 | ~100% | 37 | |
| ofloxacin | 27.7 | PMS | 2 | 72∶1 | Co(Ⅱ)/TiO2 | 500 mg/L | 3~9 | 25 | 0.5 | 73.20 | 100% | 37 | |
| Organic dyes | rhodamine B | 104.4 | PMS | 2 | 19∶1 | Co(Ⅱ)/TiO2 | 500 mg/L | 3~9 | 25 | 0.6 | — | ~100% | 37 |
| acid orange 7 | 56.9 | PMS | 0.55 | 10∶1 | Mn-Fe/LDH | 200 mg/L | 5~9 | 25 | 0.5 | 8.28 | 97.6% | 45 | |
| p-chloronitrobenzene | 2697 | PDS | 200 | 74∶1 | ZVI | 1 mmol/g | 3~6.8 | 25 | 8 | — | 90% | 46 | |
| Other pollutants | bisphenol A | 100 | PMS | 2 | 20∶1 | Mn/Fe3O4 | 200 mg/L | 7~11 | 15 | 0.5 | 43.20 | 100% | 47 |
| p-nitrophenol | 359.4 | PDS | 9 | 25∶1 | CuFe2O4 | 30 000 mg/L | 6 | 25 | 1 | — | 89% | 48 | |
| benzotriazole | 167.9 | PDS | 5 | 30∶1 | Cu(Ⅱ)/V2O5 | 200 mg/L | 9 | — | 2 | — | 97% | 35 |
a Phenanthrene is a type of polycyclic aromatic hydrocarbons(PAHs), which heretofore haven't been added to the POPs list in the Stockholm Convention. |
2.4 碳质材料活化法
2.5 碱活化法
2.6 UV活化法
表2 UV活化PS降解有机污染物Table 2 Degradation of organic pollutants by UV activated PS |
| Class | Pollutant | Conc. (μM) | PS | Conc. (μM) | Wavelength(nm) | pH | t(h) | Degradation rate | ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| Anodyne | antipyrine | 26.5 | PDS | 520 | 19.6∶1 | 253.7 | 7.2 | 1.00 | 80% | 69 |
| Antihypertensive drugs | atenolol | 20 | PMS | 80 | 4∶1 | 254 | 7 | 0.50 | ~80% | 70 |
| Antibiotics(sulfonamides) | sulfadiazine | 20 | PDS | — | — | — | 6.5 | 0.30 | 97% | 71 |
| Antibiotics (fluoroquinolones) | ciprofloxacin norfloxacin levofloxacin | 40 | PDS | 500 | 12.5∶1 | 254 | 6.5 | 0.16 | 42% 25% 1% | 67 |
| Antibiotics(β-lactams) | cephalexin oxacillin | 40 | PDS | 500 | 12.5∶1 | 254 | 6.5 | 0.16 | 84% 88% | 67 |
| POPs | endosulfan | 2.45 | PDS | 49 | 20∶1 | 254 | 7 | 1.30 | 90% | 25 |
2.7 电化学活化法
表3 各种PS活化方法的优缺点Table 3 Advantages and disadvantages of different PS activation methods |
| Activation method | Advantages | Disadvantages |
|---|---|---|
| Thermal | easy operation; no chemicals addition | high cost; poor stability |
| Mechanochemical | rapid reaction; no chemicals addition | suitable for solid pollutants |
| Transition metal | high degradation efficiency; easy operation; no special device and low energy consumption | metal ions leaching; poor stability and efficiency; high cost for large-scale remediation |
| Alkali | suitable for pollutants that can react with ·OH | restricted by pH; equipment corrosion; precipitation of metal ions |
| UV | safe ; no secondary pollution | poor reusability; high energy consumption; high requirements for PS concentration |
| Carbonaceous materials | high degradation efficiency; cheap and easy to get | restricted by pH; poor stability |
| Electrochemical | economic and environmental friendly; controllable process; high degradation efficiency | pretreatment needed; electrode passivation |
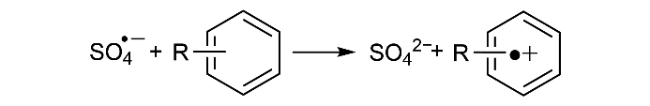
3 SO4.-降解有机污染物
3.1 POPs
表4 SO4.-降解POPsTable 4 Degradation of POPs by SO4.- |
| Class | POPs | Conc. (μM) | PS | Conc. (μM) | Activator | Conc. | pH | T(℃) | t(h) | kobs() | Degradation rate | ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OCPs | endosulfan | 2.45 | PDS | 49 | 20∶1 | UV | — | 7 | 25 | 1.3 | — | 90% | 25 |
| lindane | 3.43 | PMS | 250 | 73∶1 | UV/Fe2+ | 0.05 mM | 3 | 25 | 0.6 | 5.05 | 98% | 87 | |
| DDT | 2.82 | PMS | 2000 | 710∶1 | Co2+ | 40 mM | 3~5 | 40 | 2 | 21.60 | 100% | 102 | |
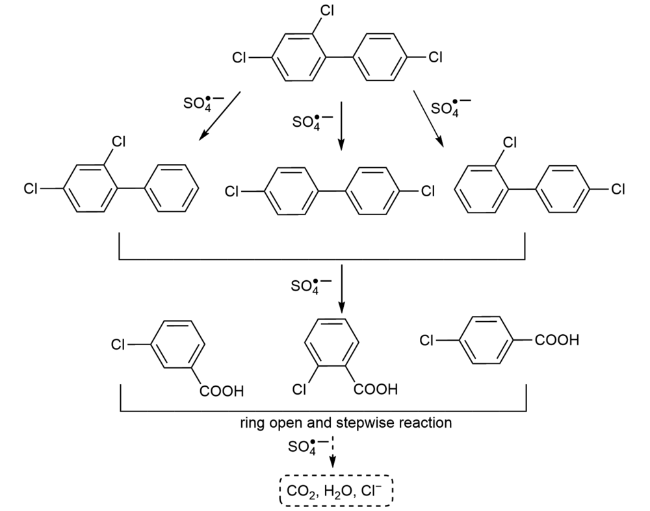
| PCBs | CB-28 | 3.9 | PDS | 2000 | 513∶1 | V2O3 | 1.33 mM | 5.9 | 25 | 4 | 0.89 | 100% | 98 |
| CB-28 | 1.94 | PMS | 200 | 103∶1 | CuFe2O4 | 0.42 mM | 7 | 25 | 8 | 0.49 | 89% | 96 | |
| CB-1 | 20 | PMS | 400 | 20∶1 | Fe2+ | 0.2 mM | 3 | 25 | 4 | 1.31 | 90% | 103 | |
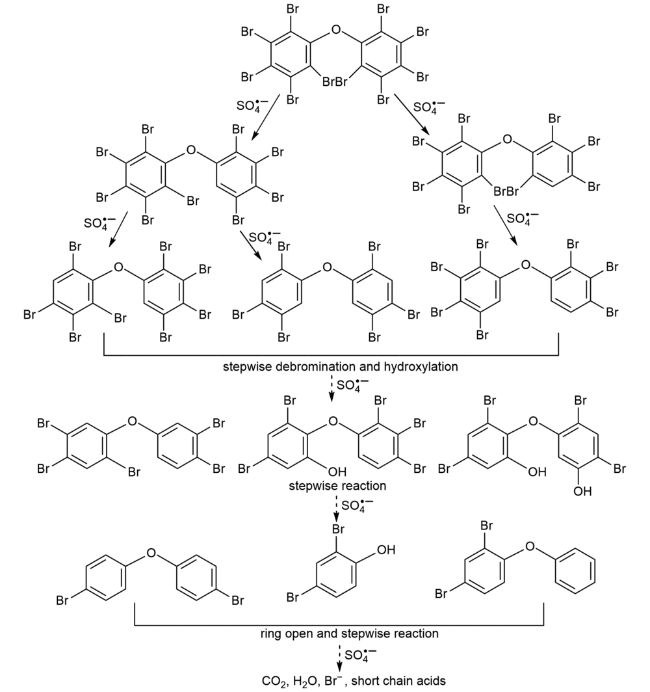
| PBDEs | BDE-47 | 3.1 | PDS | 71 400 | 23 032∶1 | nZVI | 1000 mg/L | 6~11 | 25 | 48 | — | 64% | 39 |
| BDE-209 | 5.2 | PDS | 100 000 | 19 183∶1 | BC-nZVI | 200 mM | 3 | 40 | 4 | 0.30 | 82% | 50 | |
| BDE-209 | 10.4 | PDS | 200 000 | 19 183∶1 | Fe2+ | 100 mM | 3 | 25 | 4 | 0.18 | 51% | 93 | |
| PFASs | PFOA | 120 | PDS | 12 000 | 100∶1 | AC-Fe | 1000 mg/L | 2.5 | 25 | 10 | 0.21 | 64% | 95 |
| PFOA | 0.5 | PDS | 20 000 | 40 000∶1 | heat | — | 2 | 85 | 8 | 0.83 | 98% | 99 | |
| PFOS | 0.92 | PDS | 84 000 | 91 304∶1 | heat | — | — | 90 | 70 | — | 0% | 100 | |
| PAHs | phenanthrene | 5.6 | PDS | 2000 | 357∶1 | V2O3 | 100 mg/L | 3~5 | 25 | 3.6 | 7.15 | 88% | 42 |
| Intermediate | p-chloroaniline | 500 | PDS | 2500 | 5∶1 | AC | 5000 mg/L | 3~9 | 20 | 0.5 | — | 98% | 104 |
3.2 PPCPs
表5 SO4.-降解抗生素Table 5 Degradation of antibiotics by SO4.- |
| Class | Antibiotic | Conc. (mM) | PS | Conc. (mM) | MPS∶ Pollutant | Activator | Conc. | pH | T(℃) | t(h) | kobs(h-1) | Degradation rate | ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluoroquinolones | ciprofloxacin | 0.04 | PDS | 0.5 | 12.5∶1 | UV254 | — | 6.5 | — | 0.16 | — | 70% | 67 |
| norfloxacin | 0.03 | PDS | 4.4 | 147∶1 | BC | 800 mg/L | 6.5 | 25 | 5 | 0.57 | ~100% | 56 | |
| Sulfonamides | sulfathiazole | 0.10 | PDS | 5 | 50∶1 | Cu2+ | 0.5 mM | 7 | 25 | 72 | 0.04 | 95% | 114 |
| sulfamethazine | 0.18 | PMS | 1 | 5.6∶1 | CuCo2O4 | 100 mg/L | 5 | 25 | 0.5 | 8.46 | 95% | 111 | |
| Tetracyclines | tetracycline | 0.34 | PDS | 11.1 | 32.9∶1 | Fe | 3500 mg/L | 3.6 | — | 3 | — | 81.6% | 115 |
| β-Lactams | cephalosporin | 0.10 | PDS | 1.1 | 11∶1 | Cu2+ | 0.05 mM | 7 | — | 2 | — | ~100% | 26 |
3.3 有机染料
表6 SO4.-降解有机染料Table 6 Degradation of organic dyes by SO4.- |
| Organic dye | Conc. (mM) | PS | Conc. (mM) | MPS/Pollutant | Activator | Conc. | pH | T(℃) | t(min) | kobs(h-1) | Degradation rate | ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acid orange 7 | 0.2 | PDS | 4 | 20∶1 | CoMn2O4/rGO | 50 mg/L | 7 | 25 | 20 | — | 100% | 52 | ||||
| Acid orange 7 | 0.06 | PMS | 0.55 | 9.7∶1 | Mn-Fe/LDH | 200 mg/L | 5~9 | 25 | 30 | 8.28 | 98% | 45 | ||||
| Acid orange G | 0.11 | PMS | 0.28 | 2.5∶1 | Co-Mn/LDH | 25 mg/L | 3~10 | 25 | 4 | 50.40 | 100% | 118 | ||||
| Methylene blue | 0.16 | 1.8∶1 | — | 100% | ||||||||||||
| Rhodamine B | 0.10 | 2.7∶1 | — | 100% | ||||||||||||
| Methyl orange | 0.15 | 1.8∶1 | — | 100% | ||||||||||||
| p-Chloronitrobenzene | 2.7 | PDS | 200 | 74.1∶1 | ZVI | 1.0 mmol/g | 3~7 | 25 | 480 | — | 90% | 46 | ||||