 PDF(11779 KB)
PDF(11779 KB)


 PDF(11779 KB)
PDF(11779 KB)
 PDF(11779 KB)
PDF(11779 KB)
超分子组装体系中基于三重态-三重态湮灭的上转换发光
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Triplet-Triplet Annihilation-Based Upconversion in Supramolecular System
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}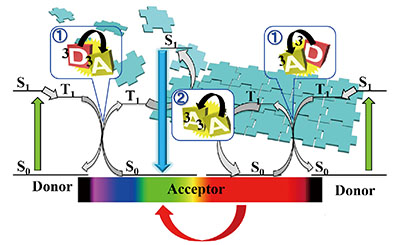
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |