

文章编号: 190922
文献标识码: A
固态荧光碳点的制备
收稿日期:2019-09-18
修回日期:2019-12-16
网络出版日期:2020-02-20
基金资助
山西省三晋学者计划、山西省高等学校中青年拔尖创新人才支持计划、山西省重点研发计划-国际合作项目(201903D421082)
山西省三晋学者计划、山西省高等学校中青年拔尖创新人才支持计划、山西省重点研发计划-国际合作项目(201803D421091)
山西省高校成果转化培育项目()
版权
Preparation of Solid-State Fluorescent Carbon Dots
Received:18 Sept. 2019
Revised:16 Dec. 2019
Online:20 Feb. 2020
Fund
Specialized Research Fund for Sanjin Scholars Program of Shanxi Province, the Program for the Innovative Talents of Higher Education Institutions of Shanxi, the Key Research and Development Plan(International Cooperation) of Shanxi Province(201903D421082)
Specialized Research Fund for Sanjin Scholars Program of Shanxi Province, the Program for the Innovative Talents of Higher Education Institutions of Shanxi, the Key Research and Development Plan(201803D421091)
Transformation of Scientific and Technological Achievements Programs of Higher Education Institutions in Shanxi (TSTAP), China()
Copyright
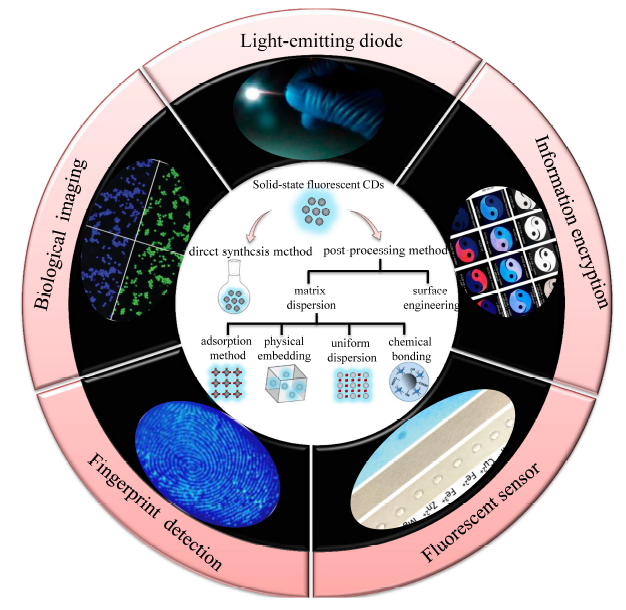
荧光碳点由于其具有无毒、制备成本低以及独特的光致发光性能而引起人们极大的研究兴趣,但是通常碳点的制备和使用均是在溶液中,而且随着碳点浓度的增加其荧光强度可能会降低甚至猝灭,通过简单干燥后得到的固态粉末则常常缺少荧光性质。因此,固态荧光碳点制备及其相关应用的研究相对较少。本文综述了固态荧光碳点的制备方法,包括后处理法(基质分散法、表面工程法)和前驱体直接合成法;对比了各种调控手段处理前后碳点荧光性能的变化情况,总结了各种固态碳点在制备过程中和使用过程中存在的主要问题。最后,针对固态发光碳点的制备方法、性能调控及发展方向进行了展望。开发具有聚集诱导发射增强的碳点是至关重要的,也为固态碳点的发展提供了新思路。
李世嘉 , 庞尔楠 , 郝彩红 , 蔡婷婷 , 胡胜亮 . 固态荧光碳点的制备[J]. 化学进展, 2020 , 32(5) : 548 -561 . DOI: 10.7536/PC190922
Shijia Li , Ernan Pang , Caihong Hao , Tingting Cai , Shengliang Hu . Preparation of Solid-State Fluorescent Carbon Dots[J]. Progress in Chemistry, 2020 , 32(5) : 548 -561 . DOI: 10.7536/PC190922
Fluorescent carbon dots have attracted significant interest for their non-toxicity, low cost and unique photoluminescence properties. Generally, the preparation and usage of carbon dots(CDs) are in solution. With the increase of CDs concentration, their fluorescence intensity may be reduced or even quenched. Following, the solid-state fluorescent CDs powder obtained by simple drying often lack of fluorescence properties. Therefore, there are relatively few researches on the preparation and related applications of solid-state fluorescent CDs. The article describes recent preparation methods of solid-state fluorescent CDs, including post-processing methods(matrix dispersion method, surface engineering) and direct synthesis method. The changes of fluorescence properties of CDs before and after treatment are compared, and the main problems in preparation and application of solid CDs are summarized. Meanwhile, the preparation, performance modulation of solid-state fluorescent CDs are prospected. It is very crucial to exploit the CDs with the enhancement of aggregation induced emission, which provides a new strategy for the development of solid-state fluorescence CDs.
1 Introduction
2 Preparation of solid-state fluorescent carbon dots by post-processing method
2.1 Matrix dispersion method
2.2 Surface engineering method
3 Preparation of solid-state fluorescent carbon dots by direct synthesis method
4 Conclusion
图2 质量比为1∶450、1∶70、1∶20的CDs@淀粉粉末在日光下(a)和紫外光下(b)图像;质量比为1∶70的CDs@淀粉粉末在紫外(c和c')、蓝光(d和d')、绿光(e和e')激发下的荧光图像[63] Fig. 2 Photographs of CDs@starch powder with a mass ratio of 1∶450,1∶70,1∶20 under sunlight (a) and ultraviolet (b). Fluorescence photographs of CDs@starch powder with a mass ratio of 1∶70 excited by ultraviolet(c and c'), blue light(d and d'), and green light(e and e') [63]. Copyright 2014, Royal Society of Chemistry |
图6 F-CDs@SrCO3 (a)和F-CDs@BaCO3 (d)的SEM;F-CDs@SrCO3(b, c), F-CDs@BaCO3 (e, f),F-CDs@CaSO4 ·2H2O(g, h) 和F-CDs@SrSO4(j, k)的光学显微图像(b, e, g, j)和共聚焦荧光显微镜图像(c, f, h, k);F-CDs@CaSO4 ·2H2O(i) 和 F-CDs@SrSO4 (l)的分布模型;(m) 9种F-CDs/无机纳米复合材料在紫外线激发和去除紫外线激发后的照片;Ca、Sr和Ba的碳酸盐(n)、硫酸盐(o)和草酸盐(p)的 F-CDs/无机物纳米复合材料的稳态光致发光光谱[69]Fig. 6 SEM of F-CDs @SrCO3(a) and F-CDs@BaCO3(d). Optical microscopy images(b, e, g, j) and confocal fluorescence microscopy images(c, f, h, k) of F-CDs@SrCO3(b, c), F-CDs @BaCO3(e, f),F-CDs@CaSO4 ·2H2O(g, h) and F-CDs@SrSO4(j, k).Distribution models of F-CDs@CaSO4 ·2H2O(i) and F-CDs@SrSO4(l).(m)Photographs of the nine F-CDs/inorganic nanocomposites upon UV excitation and after removal of UV excitation. Steady-state photoluminescence emission spectra of Ca, Sr and Ba carbonates(n), sulphates(o) and oxalates(p) F-CDs/inorganic nanocomposites[69]. Copyright 2019, Nature |
图8 (a,b)CDs水溶液和Zr-MOF的激发和发射光谱图;(c) 在365 nm激发下CDs、Zr-MOF、CDs@Zr-MOF荧光发射图;(d)CDs、Zr-MOF和CDs@Zr-MOF荧光衰变图[71]Fig. 8 (a,b)Excitation and emission spectra of CDs aqueous solution and Zr-MOF.(c) PL emission spectra of CDs, Zr-MOF and CDs@Zr-MOF excited under 365 nm.(d)PL decays of CDs(blue), Zr-MOF(red) and CDs@Zr-MOF(black)[71]. Copyright 2019, Royal Society of Chemistry |
表1 各种前驱体和制备方法得到CDs的光学性能及其应用Table 1 Summary of optical properties and applications of the CDs from various precursors and preparation methods |
| Precursor | Method | Synthesized CDs | Size/nm | Ex/nm | Em/nm | QY/% | Application | ref | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA Urea starch | water(MW, 750 W, 5 min) chemical adsorption | g-CDs CDs@starch (mass ratio:1:70) | 2~20[89] 20~40 (μm) | 420 420 | 540 515 | 18 50 | LEDs, temperature sensors | 63 | |||||||
| CA Urea BaCl2,Na2SO4 | water(MW, 750 W, 5 min) electrostatic adsorption | g-CDs CDs@BaSO4 | 2~20 60~150 | 405 405 | 522 520 | 17 27 | LEDs | 64 | |||||||
| CA, ethylenediamine Zn(Ac)·2H2O, KOH APTES | water(300 ℃, 5 h) stirring electrostatic adsorption | CDs ZnO CDs-ZnO@APTES | 2~10 4~6 2~10 | 300 370 365 | 450 540 450~540 | 49 | WLEDs | 65 | |||||||
| AAPMS, CA KBr,KCl,NaCl | (240 ℃, 1 h) physical embedding | CDs CDs@salt | 360 360 | 440 440 | LEDs | 58, 66 | |||||||||
| CA, Urea NaCl | water(MW, 750 W, 5 min) physical embedding | g-CDs CDs@NaCl | 2~5 2~5 (μm) | 405 405 | 522 510 | 14 25 | WLEDs | 67 | |||||||
| CA, L-cysteine TMA-POSS | water(200 ℃, 3 h) physical embedding | CDs CDs@TMA-POSS (2×105:1) | 3.0~6.5 | 200~400 260~400 | 420 415 | 78 60 | solid-state lighting devices | 68, 90 | |||||||
| sodiumfolate CaCl2·2H2O,Na2CO3 SrCl2·6H2O,Na2CO3 BaCl2,Na2CO3 CaCl2·2H2O,Na2SO4 SrCl2·6H2O,Na2SO4 BaCl2,Na2SO4 CaCl2·2H2O,Na2C2O4 SrCl2·6H2O,Na2C2O4 BaCl2,Na2C2O4 | water(200 ℃, 12 h) | F-CNDs F-CNDs@CaCO3 F-CNDs@SrCO3 F-CNDs@BaCO3 F-CNDs@CaSO4·2H2O F-CNDs@SrSO4 F-CNDs@BaSO4 F-CNDs@CaC2O4·H2O F-CNDs@SrC2O4·H2O F-CNDs@BaC2O4·0.5H2O | 3~5 | 320 320 320 320 320 320 320 320 320 320 | 398 398 398 398 398 398 398 398 398 398 | 11 6.8 3.2 0.5 7.2 1.7 0.0 2.7 0.3 0.6 | theranostic agents forbackgroundless bio-imaging pH-responsive controlled- release materials | 69 | |||||||
| Precursor | Method | Synthesized CDs | Size/nm | Ex/nm | Em/nm | QY/% | Application | ref | |||||||
| PPDA KH-792 | ethanol(180 ℃,6 h) physical embedding | CDs CDs@silica powder | 4.0~9.0 | 365~525 385~525 | 600 597 | 52.46 41.72 | LEDs | 70 | |||||||
| CA, urea H4L, benzoic acid ZrOCl2·8H2O, DMF AEATMS | ammonia water (MW,700 W,6 min) (120 ℃,72 h) | CDs CDs@Zr-MOF (dispersed in AEATMS) | 4 | 365 365 | 450 450 511 550 | 22 37 | WLEDs | 71 | |||||||
| ethylene glycol DMF | (200 ℃, 5 h) ethylenediamine modification surface functionalization | N-CDs CDs@DMF (VN-CDs/VDMF :0.25) | 1~5 | 445 445 | new devices and materials | 73, 91 | |||||||||
| CA SBA-15 | water(200 ℃, 5 h) ammonia solution (200 ℃,5 h) surface functionalization | CDs CDs@SBA-15 | 3.0 9.5 | 400 340 | 480 410 | sensing | 74, 92 | ||||||||
| CA NH2-POSS | water(200 ℃, 5 h) surface functionalization | CDs CDs@NH2-POSS | 2~7 2~9 | 300~380 300~380 | 445 450 | 6.4 10.2 | composite fillers | 75, 93 | |||||||
| CA H2O2 | ammonia water (MW,650 W,5 min) (70 ℃,2 h) surface functionalization | CDs Ox-CDs Ox-CDs powder | 2~4 2~4 2~4 | 330~370 340~370 270~500 | 435 435 520 | 21 17 25 | solid-state lightning, high-speed VLC, LEDs | 76 | |||||||
| PVA,EDA PVA PVA,DETA PVA,TEPA | water(220 ℃, 10 h) water(220 ℃, 10 h) water(220 ℃, 10 h) water(220 ℃, 10 h) | CDs220 aqueous solution CDs220 powder PVA220 aqueous solution PVA220 powder d-CDs220 aqueous solution d-CDs220 powder t-CDs220 aqueous solution t-CDs220 powder | 9 | 340 340 365 350 365 360 365 | 540 460 450 580 470 550 | 35 1 20 22 | LEDs | 78 | |||||||
| Tween 80 | phosphoric acid, sulfuric acid(90 ℃, 3 h) one-step carbonization | CDs(CH2Cl2) CDs powder | 3.5~5.3 | 363 365 | 435 455 | 2.1 2.0 | visualizationoffing-erprints,LEDs | 79 | |||||||
| trisodium citrate dehydrate urea | DMF(160 ℃, 4 h) In-situ embedding DMAC(160 ℃, 4 h) In-situ embedding DEF(160 ℃, 4 h) In-situ embedding | CDs11 aqueous solution CDs11powder CDs12 aqueous solution CDs12powder CDs21 aqueous solution CDs21powder | 400 100~500 300~500 | 422 422 412 412 414 414 | 537 530 513 | 20.8 21.6 14.9 18.7 17.5 17.6 | WLEDs, fluorescent plates | 80 | |||||||
| Al(OiPr)3 H3PO4 HF Al(OiPr)3 H3PO4 | trimethylamine triethylene glycol (180 ℃, 3 days) 4,7,10-trioxa-1, 13-tridecanediamine triethylene glycol (180 ℃, 3 days) | CDs@AlPO-5 CDs@2D-AlPO | 3.7 nm 3.5 nm | 370 370 | 430 440 | 15.53 52.14 | smart material in dual-mode security protection | 81 | |||||||
| Al(OiPr)3 H3PO4 | MgHPO4 ·3H2O,H2O 4,7,10-trioxa-1, 13-tridecanediamine (180 ℃, 3 days) In-situ embedding | CDs@MgAPO-5 | 3.4 nm | 370 | 425 | 22.77 | |||||||||
| MA,DTSA | acetic acid (180 ℃,10 h) | H-CDs(acetic acid) H-CD powder | 4~10 4~10 | 360 559 | 467 620 | 5.96 | luminescence ink, encryption tool | 82 | |||||||
| CA,Urea,CaCl2 | vacuum heating | v-CDs(ethanol solution) | 4.1 | 380~430 | 510~514 | 72 | encryption medium | 83 | |||||||
| CA,L-cysteine KCl | one-pot microwave heating(5 min) | CDs solution (0.2 mg·mL-1) CDs powder | 2.1 | 340~380 430~500 | 435 500~620 | 84 65 | WLEDs | 84 | |||||||
图9 (a,b)固态组装的SEM图像;(c)网状沉淀的SEM,(d)N-CD溶液、固态组装、网状沉淀和不含N-CDs粉末的荧光谱;(e)席夫碱形成原理图[73]Fig. 9 (a,b) SEM images of the solid assemblies.(c) SEM image of the ramified precipitates.(d) PL spectra of N-CD’s solution, solid assemblies, ramified precipitates and powders without N-CDs.(e) Schematic diagram of Schiff base formation[73]. Copyright 2015, Royal Society of Chemistry |
图10 (a)CDs@SBA-15形成原理图;(b)NCDs和NCDS@SBA-15的荧光激发和发射谱;(c)NCDs@SBA-15在水、浓盐酸和氨水溶液中的荧光谱(λex=340 nm);(d)CDs@SBA-15在紫外灯下照片[74]Fig. 10 (a) Schematic diagram of CDs@SBA-15, (b) PL excitation and emission spectra of NCDs and NCDs@SBA-15.(c) PL spectra of NCDs@SBA-15 in water, concentrated HCl and ammonia solution(λex=340 nm).(d) Photographs of CDs@SBA-15 under UV light[74]. Copyright 2019, Royal Society of Chemistry |
图12 (a),(b)CDs220乙醇溶液的TEM图;(c)不同浓度CDs220水溶液和CDs220粉末在340 nm激发下的荧光发射图谱;(d)d-CDs220(1),t-CDs220(2),CDs220(3),PVA220(4)和CDs220@淀粉(5)在日光(上)和紫外光(下)的图像;(e)以上粉体的固态荧光图谱[78]Fig. 12 (a), (b) TEM images of CDs220 ethanol solution.(c) Fluorescence emission spectra of CDs220 aqueous solution and CDs220 powders excited at 340 nm.(d) Images of d-CDs220(1), t-CDs220(2), CDs220(3), PVA220(4) and CDs220@starch(5) under daylight(top) and ultraviolet(bottom). (e) Solid-state fluorescence spectra of the above powders[78]. Copyright 2015, Wiley |
图15 (a) CDs@AlPO-5复合材料的SEM图(左)和在紫外、蓝光和绿光激发下的荧光图(右);(b)CDs@2D-AlPO复合材料的SEM图(左)和在紫外、蓝光和绿光激发下的荧光图(右);(c)CDs@MgAPO-5复合材料的SEM图(左)和在紫外、蓝光和绿光激发下的荧光图(右);(d)CDs@AlPO-5在室温下的荧光衰变图[81]Fig. 15 (a)SEM image(left) and fluorescence microscopy images(right) excited under UV, blue and green light of CDs @AlPO-5 composite.(b)SEM image(left) and fluorescence microscopy images(right) excited under UV, blue and green light of CDs@2D-AlPO composite.(c)SEM image(left) and fluorescence microscopy images(right) excited under UV, blue and green light of CDs@MgAPO-5.(d)PL decays of CDs@AlPO-5 at room temperature[81]. Copyright 2017, Science |
图16 (a)蓝色H-CDs单体和红色团聚体;(b)H-CDs从分散到团聚体形成原理图;(c)蓝色荧光猝灭和红色荧光打开原理(左)和H-CDs的表面和核心结构(右)[82]Fig. 16 (a) The schematic diagram of blue H-CDs monomers and red aggregates.(b) The schematic diagram of H-CDs formation from dispersion to aggregate.(c) Principle of blue fluorescence quenching and red fluorescence opening(left) and proposed surface and core structure of H-CDs(right)[82]. Copyright 2019,Nature |
| [1] |
Sun Y P, Zhou B, Lin Y, Wang W, Shiral F K A, Pankaj P, Mohammed J M, Barbara A H, Wang X, Wang H F, Luo P G, Yang H, Muhammet E K, Chen B L, Veca L M, Xie S Y. Journal of the American Chemical Society, 2006,128:7756. https://pubs.acs.org/doi/10.1021/ja062677d
DOI: 10.1021/ja062677d |
| [2] |
Bhattacharyya S, Ehrat F, Urban P, Teves R, Wyrwich R, Doblinger M, Feldmann J, Urban A S, Stolarczyk J K. Nature Communications, 2017,8(1):1401. http://www.nature.com/articles/s41467-017-01463-x
|
| [3] |
Li W D, Liu Y, Wu M, Feng X L, Redfern S A T, Shang Y, Yong X, Feng T L, Wu K F, Liu Z Y, Li B J, Chen Z M, Tse J S, Lu S Y, Yang B. Advanced Materials, 2018,30(31):1800676. http://doi.wiley.com/10.1002/adma.v30.31
DOI: 10.1002/adma.v30.31 |
| [4] |
Liu C A, Fu Y J, Xia Y J, Zhu C, Hu L L, Zhang K, Wu H H, Huang H, Liu Y, Xie T F, Zhong J, Kang Z H. Nanoscale, 2018,10(5):2454. http://xlink.rsc.org/?DOI=C7NR08000J
DOI: 10.1039/C7NR08000J |
| [5] |
Zhang J Y, Wu S H, Lu X M, Wu P, Liu J W. Nano Letters, 2019,19(5):3214 https://pubs.acs.org/doi/10.1021/acs.nanolett.9b00725
|
| [6] |
金静(Jin J), 朱守俊(Zhu S J), 宋玉彬(Song Y B), 宋薇(Song W), 杨柏(Yang B), 赵冰(Zhao B). 光谱学与光谱分析 (Spectroscopy and Spectral Analysis), 2016,36:291.
|
| [7] |
白静静(Bai J J), 胡国胜(Hu G S), 张静婷(Zhang J T), 刘冰肖(Liu B X), 王玉龙(Wang Y L), 李振中(Li Z Z). 光子学报 (Acta Photonica Sinica), 2019,48(4):0416001
|
| [8] |
Jiang K, Sun S, Zhang L, Wang Y H, Cai C Z, Lin H W. ACS Applied Materials & Interfaces, 2015,7(41):23231. https://pubs.acs.org/doi/10.1021/acsami.5b07255
|
| [9] |
Miao X, Yan X L, Qu D, Li D B, Tao F F, Sun Z C. ACS Applied Materials & Interfaces, 2017,9(22):18549. https://pubs.acs.org/doi/10.1021/acsami.7b04514
|
| [10] |
Chen J, Wei J S, Zhang P, Niu X Q, Zhao W, Zhu Z Y, Ding H, Xiong H M. ACS Applied Materials & Interfaces, 2017,9(22):18429. https://pubs.acs.org/doi/10.1021/acsami.7b03917
|
| [11] |
曲松楠(Qu S N), 刘星元(Liu X Y), 申德振(Shen D Z). 发光学报 (Chinese Journal of Luminescence), 2014,35:1019.
|
| [12] |
Liu J J, Li D W, Zhang K, Yang M X, Sun H C, Yang B. Small, 2018,14(15):1703919. http://doi.wiley.com/10.1002/smll.201703919
|
| [13] |
Yang L, Jiang W H, Qiu L P, Jiang X W, Zuo D Y, Wang D K, Yang L. Nanoscale, 2015,7(14):6104. http://xlink.rsc.org/?DOI=C5NR01080B
DOI: 10.1039/C5NR01080B |
| [14] |
Yang W N, Zhang H, Lai J X, Peng X Y, Hu Y P, Gu W, Ye L. Carbon, 2018,128:78. https://linkinghub.elsevier.com/retrieve/pii/S0008622317311831
|
| [15] |
Strauss V, Marsh K, Kowal M D, El-Kady M, Kaner R B. Advanced Materials, 2018,30(8):1704449. http://doi.wiley.com/10.1002/adma.v30.8
DOI: 10.1002/adma.v30.8 |
| [16] |
Wang F, Chen Y H, Liu C Y, Ma D G. Chemical Communications, 2011,47(12):3502. http://dx.doi.org/10.1039/c0cc05391k
DOI: 10.1039/c0cc05391k We demonstrate the first white light-emitting device originating from single carbon dot components. A maximum external quantum efficiency of 0.083% at a current density of 5 mA cm(-2) with a color-rendering index of 82 is realized, indicating that carbon dots have great potential to be an alternative phosphor for fabricating white light electroluminescent devices. |
| [17] |
Zhang D Z, Liu C Y, Li K Z, Chen Y, Ruan S P, Zhang X D, Li C N. Nanoscale, 2018,10(14):6459. http://xlink.rsc.org/?DOI=C8NR00214B
DOI: 10.1039/C8NR00214B |
| [18] |
Li L, Chen Y H, Liu Z H, Chen Q, Wang X D, Zhou H P. Advanced Materials, 2016,28(44):9862 http://doi.wiley.com/10.1002/adma.201603021
|
| [19] |
Sun C, Zhang Y, Ruan C, Yin C Y, Wang X Y, Wang Y D, Yu W W. Advanced Materials, 2016,28(45):10088. http://doi.wiley.com/10.1002/adma.201603081
|
| [20] |
Wang Y L, Yan L P, Ji G Q, Wang C, Gu H M, Luo Q, Chen Q, Chen L W, Yang Y Z, Ma C Q, Liu X G. ACS Applied Materials & Interfaces, 2019,11(2):2243. https://pubs.acs.org/doi/10.1021/acsami.8b17128
|
| [21] |
Hu S L, Guo Y, Dong Y G, Yang J L, Liu J, Cao S R. Journal of Materials Chemistry, 2012,22(24):12053. http://dx.doi.org/10.1039/c2jm30584d
DOI: 10.1039/c2jm30584d The effects of the structures on the energy gaps in luminescent carbon nanoparticles (CNPs) were investigated. On the one hand, we fabricated CNPs with the different structures and a uniform size and then analyzed their photoluminescence (PL) behaviors. On the other hand, we calculated the dependence of the structures on the energy gaps in CNPs by a simple quantitative model. Both the experimental and calculated results show that the luminescent CNPs contain a mixture of sp(2) and sp(3) bonding and hence their PL behaviors and energy gaps are determined by the fraction of sp(2)-hybridized carbon atoms. |
| [22] |
Hu S L, Dong Y G, Yang J L, Liu J, Tian F, Cao S R. Asian Journal of Chemistry, 2012,7(11):2711.
|
| [23] |
Ray S C, Saha A, Nikhil R J, Rupa S. The Journal of Physical Chemistry C, 2009,113:18546. https://pubs.acs.org/doi/10.1021/jp905912n
DOI: 10.1021/jp905912n |
| [24] |
Tian L, Ghosh D, Chen W, Pradhan S, Chang X J, Chen S W. Chemistry of Materials, 2009,21(13):2803. https://pubs.acs.org/doi/10.1021/cm900709w
DOI: 10.1021/cm900709w |
| [25] |
Hu C, Yu C, Li M Y, Wang X N, Yang J Y, Zhao Z B, Eychmuller A, Sun Y P, Qiu J S. Small, 2014,10(23):4926. http://dx.doi.org/10.1002/smll.201401328
The desired control of size, structure, and optical properties of fluorescent carbon dots (CDs) is critical for understanding the fluorescence mechanism and exploring their potential application. Herein, a top-down strategy to chemically tailor the inexpensive coal to fluorescent CDs by a combined method of carbonization and acidic oxidation etching is reported. The size and optical properties of the as-made CDs are tuned by controlling the structures of graphitic crystallites in the starting precursor. The coal-derived CDs exhibit two different distinctive emission modes, where the intensity of the short-wavelength emission is significantly enhanced by partial reduction treatment. The evolution of the electronic structure and the surface states analysis show that two different types of fluorescence centers, nano-sized sp(2) carbon domains and surface defects, are responsible for the observed emission characteristics. The reduced CDs are demonstrated as an effective fluorescent sensing material for label-free and selective detection of Cu(II) ions with a detection limit as low as 2.0 nM, showing a great promise for real-world sensor applications. |
| [26] |
Deng J H, Lu Q J, M N X, Li H T, Liu M L, Xu M C, Tan L, Xie Q J, Zhang Y Y, Yao S Z. Chemistry, 2014,20(17):4993.
|
| [27] |
Li H T, He X D, Kang Z H, Huang H, Liu Y, Liu J L, Lian S Y, Tsang C H, Yang X B, Lee S T. Angewandte Chemie International Edition, 2010,49(26):4430. http://doi.wiley.com/10.1002/anie.200906154
|
| [28] |
Zhang Y L, Wang L, Zhang H C, Liu Y, Wang H Y, Kang Z H, Lee S T. RSC Advances, 2013,3(11):3733. http://dx.doi.org/10.1039/c3ra23410j
DOI: 10.1039/c3ra23410j Reported here is a green synthesis of graphitic carbon quantum dots (GCQDs) as a fluorescent sensing platform for the highly sensitive and selective detection of Fe3+ ions. Through the electrochemical ablation of graphite electrodes in ultrapure water, uniform GCQDs with graphitic crystallinity and oxygen containing groups on their surfaces have been successfully prepared. The absence of acid, alkali, salt and organic compounds in the starting materials effectively avoids complex purification procedures and environmental contamination, leading to a green and sustainable synthesis of GCQDs. The oxygen functional groups (e. g., hydroxyl, carboxyl) contribute to the water solubility and strong interaction with metal ions, which enable the GCQDs to serve as a fluorescent probe for the highly sensitive and selective detection of Fe3+ ions with a detection limit as low as 2 nM. The high sensitivity of our GCQDs could be attributed to the formation of complexes between Fe3+ ions and the phenolic hydroxyls of GCQDs. The fluorescence lifetime of GCQDs in the presence and absence of Fe3+ was tested by time-correlated single-photon counting (TCSPC), which confirmed a dynamic fluorescence quenching mechanism. |
| [29] |
Bao L, Zhang Z L, Tian Z Q, Zhang L, Liu C, Lin Y, Qi B P, Pang D W. Advanced Materials, 2011,23(48):5801. http://dx.doi.org/10.1002/adma.201102866
The size of C-nanodots can be electrochemically tuned by changing the applied potential during their preparation. The higher the applied potential, the smaller the resulting C-nanodots. Moreover, the surface oxidation degree of the C-nanodots can also be electrochemically tuned. The redshift of emission independent of the size provides an insight into the luminescence mechanism of C-nanodots. |
| [30] |
Guo Y M, Wang Z, Shao H W, Jiang X Y. Carbon, 2013,52:583. http://dx.doi.org/10.1016/j.carbon.2012.10.028
DOI: 10.1016/j.carbon.2012.10.028 We have developed a simple, one-step hydrothermal method for the synthesis of highly fluorescent carbon nanoparticles (F-CNPs) with a high quantum yield (68%) and good photostability. The method requires less reaction time and a lower reaction temperature as compared with the previous reported methods. The as-prepared F-CNPs exhibit excellent emission property and high stability, as well as excitation-independent emission behavior. Moreover, it is attractive that F-CNPs can be used as an effective fluorescent probe for the detection of mercury ions with good selectivity and sensitivity in an aqueous solution. (C) 2012 Elsevier Ltd. |
| [31] |
卢思宇(Lu S Y), 杨柏(Yang B). 高分子学报 (Acta Polymerica Sinica), 2017,7:1200.
|
| [32] |
Jiang K, Sun S, Zhang L, Lu Y, Wu A G, Cai C Z, Lin H W. Angew. Chem. Inter. Ed., 2015,54(18):5450.
|
| [33] |
Li D, Jing P T, Sun L H, An Y, Shan X Y, Lu X H, Zhou D, Han D, Shen D Z, Zhai Y C, Qu S N, Zboril R, Rogach A L. Advanced Materials, 2018,30(13):1705913. https://onlinelibrary.wiley.com/toc/15214095/30/13
DOI: 10.1002/adma.v30.13 |
| [34] |
Ding H, Wei J S, Zhang P, Zhou, Z Y, Gao Q Y, Xiong H M. Small, 2018,14(22):1800612. http://doi.wiley.com/10.1002/smll.v14.22
DOI: 10.1002/smll.v14.22 |
| [35] |
Yang S H, Sun X H, Wang Z Y, Wang X Y, Guo G S, Pu Q S. Nano Research, 2018,11(3):1369. https://doi.org/10.1007/s12274-017-1751-8
|
| [36] |
Qu S N, Wang X Y, Lu Q P, Liu X Y, Wang L J. Angewandte Chemie International Edition, 2012,51(49):12215. http://doi.wiley.com/10.1002/anie.v51.49
DOI: 10.1002/anie.v51.49 |
| [37] |
Wang L, Zhu S J, Wang H Y, Qu S N, Zhang Y L, Zhang J H, Chen Q D, Xu H L, Han W, Yang B, Sun H B. ACS Nano, 2014,8(3):2541. https://pubs.acs.org/doi/10.1021/nn500368m
DOI: 10.1021/nn500368m |
| [38] |
Xu X Y, Ray R, Gu Y L, Ploehn H J, Gearheart L, Raker K, Scrivens W A. J. Am. Chem. Soc, 2004,126:12736. https://pubs.acs.org/doi/10.1021/ja040082h
DOI: 10.1021/ja040082h |
| [39] |
Miao X, Qu D, Yang D X, Nie B, Zhao Y K, Fan H Y, Sun Z C. Advanced Materials, 2018,30(1):1704740. http://doi.wiley.com/10.1002/adma.201704740
|
| [40] |
胡胜亮(Hu S L), 白培康(Bai P K), 孙景(Sun J), 曹士锐(Cao S R). 化学进展 (Progress in Chemistry), 2010,22:345. http://www.progchem.ac.cn//CN/abstract/abstract10278.shtml
与其它荧光纳米粒子相比,荧光碳纳米颗粒不仅具有良好生物相容性和易于表面功能化等优点,还具有发光稳定并可实现上转换荧光发射的特性,所以在生物医药领域具有重要的应用价值。结合近年来的最新研究成果,本文综述了金刚石、石墨和非晶等不同结构的荧光碳纳米颗粒的制备方法及其局限性;分析了不同结构碳纳米颗粒的荧光发射特性和在生物技术中应用的优缺点;阐述了荧光碳纳米颗粒在今后研究中需要解决的问题和发展方向。 |
| [41] |
Wang B B, Jin J C, Xu Z Q, Jiang Z W, Li X, Jiang F L, Liu Y. Journal of Colloid and Interface Science, 2019,551:101. https://linkinghub.elsevier.com/retrieve/pii/S0021979719305259
|
| [42] |
Bao L, Liu C, Zhang Z L, Pang D W. Advanced Materials, 2015,27(10):1663. http://doi.wiley.com/10.1002/adma.201405070
|
| [43] |
Ding Y F, Zheng J X, Wang J L, Yang Y Z, Liu X G. Journal of Materials Chemistry C, 2019,7(6):1502 http://xlink.rsc.org/?DOI=C8TC04887H
DOI: 10.1039/C8TC04887H |
| [44] |
Ding H, Yu S B, Wei J S, Xiong H M. ACS Nano, 2016,10(1):484. https://pubs.acs.org/doi/10.1021/acsnano.5b05406
|
| [45] |
Yuan F L, Wang Z B, Li X H, Li Y C, Tan Z A, Fan L Z, Yang S H. Advanced Materials, 2017,29(3):1604436. http://doi.wiley.com/10.1002/adma.v29.3
DOI: 10.1002/adma.v29.3 |
| [46] |
Wang Z F, Yuan F L, Li X, Li H, Y, Zhong H Z, Fan L Z, Yang S H. Advanced Materials, 2017,29(37):1702910. https://onlinelibrary.wiley.com/toc/15214095/29/37
DOI: 10.1002/adma.v29.37 |
| [47] |
Lu S Y, Sui L Z, Liu J J, Zhu S J, Chen A, Jin M X, Yang B. Advanced Materials, 2017,29(15):1603443. http://doi.wiley.com/10.1002/adma.201603443
|
| [48] |
Ehrat F, Bhattacharyya S, Schneider J, Lof A, Wyrwich R, Rogach A L, Stolarczyk J K, Urban A S, Feldmann J. Nano Letters, 2017,17(12):7710. https://pubs.acs.org/doi/10.1021/acs.nanolett.7b03863
|
| [49] |
Krysmann M J, Kelarakis A, Dallas P, Giannelis E P. Journal of the American Chemical Society, 2012,134(2):747. http://dx.doi.org/10.1021/ja204661r
DOI: 10.1021/ja204661r We present a systematic investigation of the formation mechanism of carbogenic nanoparticles (GNPs), otherwise referred to as C-dots, by following the pyrolysis of citric acid (CA)-ethanolamine (EA) precursor at different temperatures. Pyrolysis at 180 degrees C leads to a CNP molecular precursor with a strongly intense photoluminescence (PL) spectrum and high quantum yield formed by dehydration of CA EA. At higher temperatures (230 degrees C) a carbogenic core starts forming and the PL is due to the presence of both molecular fluorophores and the carbogenic core. CNPs that exhibit mostly or exclusively PL arising from carbogenic cores are obtained at even higher temperatures (300 and 400 degrees C, respectively). Since the molecular fluorophores predominate at low pyrolysis temperatures while the carbogenic core starts forming at higher temperatures, the PL behavior of CNPs strongly depends on the conditions used for their synthesis. |
| [50] |
Essner J B, Kist J A, Polo-Parada L, Baker, G A. Chemistry of Materials, 2018,30(6):1878. https://pubs.acs.org/doi/10.1021/acs.chemmater.7b04446
|
| [51] |
Wang X, Cao L, Lu F S, Meziani M J, Li H T, Qi G, Zhou B, Harruff B A, Kermarrec F, Sun Y P. Chemical Communications, 2009,25:3774.
|
| [52] |
Hu S L, Chang Q, Lin K, Yang J L. Carbon, 2016,105:484. https://linkinghub.elsevier.com/retrieve/pii/S0008622316303499
|
| [53] |
丁艳丽(Ding Y L), 胡胜亮(Hu S L), 常青(Chang Q). 高等学校化学学报 (Chemical Journal of Chinese Universities), 2015,36:619. http://www.cjcu.jlu.edu.cn/CN/abstract/abstract25698.shtml
DOI: 10.7503/cjcu20140930 通过混合氨基修饰碳点(N-CDs)与酞菁锌(PcZn)合成了静电结合的N-CDs/PcZn复合结构. 利用荧光光谱、紫外-可见吸收光谱、循环伏安测试和光催化活性表征证实了N-CDs的光激发电子通过界面转移到了PcZn分子上, 然后在PcZn上发生辐射复合, 导致PcZn的荧光发射增强. 由于N-CDs上的激发电子转移到了PcZn分子上, 促使了其与空穴的分离, 阻碍了N-CDs上的辐射复合发生, 因此提高了N-CDs/PcZn复合体系的光催化活性. 反应温度会影响N-CDs/PcZn复合体系的稳定性和光转换能力, 在常温下制备的N-CDs/PcZn复合结构具有最佳的光物理与化学性能. |
| [54] |
Yuan Y S, Jiang J Z, Liu S P, Yang J D, Zhang H, Yan J J, Hu X L. Sensors and Actuators B: Chemical, 2017,242:545. https://linkinghub.elsevier.com/retrieve/pii/S092540051631841X
|
| [55] |
Deng Y H, Chen X, Wang F, Zhang X A, Zhao D X, Shen D Z. Nanoscale, 2014,6(17):10388. http://dx.doi.org/10.1039/c4nr02544j
DOI: 10.1039/c4nr02544j Fluorescent carbon dots (CDs) have received great research interest in recent years, with applications in areas such as bio-imaging and chemical sensing. However, solid state photoluminescence of CDs and its related applications (e. g. optoelectronics) is a less explored territory. Here, we have systematically studied the photo emission of CDs in solid state. We found that their blue emission is highly dependent on whether the environment contains polar groups or not. Mechanism studies show that the blue emission of CDs may come from their C=O bonds conjugated with aromatic carbons, and the interaction between polar groups in environment and C=O bonds in CDs is responsible for the environment-dependent photo emission. Our conclusion here should assist the development of CDs' solid state applications. |
| [56] |
Xie Z, Wang F, Liu C Y. Advanced Materials, 2012,24(13):1716. http://doi.wiley.com/10.1002/adma.201104962
|
| [57] |
Wang Y, Kalytchuk S, Zhang Y, Shi H C, Kershaw S V, Rogach A L. The Journal of Physical Chemistry Letters, 2014,5(8):1412. https://pubs.acs.org/doi/10.1021/jz5005335
DOI: 10.1021/jz5005335 |
| [58] |
Gan Z X, Liu L Z, Wang L, Luo G S, Mo C L, Chang C L. Physical Chemistry Chemical Physics, 2018,20(26):18089. http://xlink.rsc.org/?DOI=C8CP02069H
DOI: 10.1039/C8CP02069H |
| [59] |
Bhunia S K, Nandi S, Shikler R, Jelinek R. Nanoscale, 2016,8(6):3400. http://xlink.rsc.org/?DOI=C5NR08400H
DOI: 10.1039/C5NR08400H |
| [60] |
Wang W T, Kim T H, Yan Z F, Tade M O, Li Q. Advanced Materials Research, 2012,557/559:739. https://www.scientific.net/AMR.557-559
|
| [61] |
Wang Y L, Zhao Y Q, Zhang F, Chen L, Yang Y Z, Liu X G. New Journal of Chemistry, 2016,40(10):8710. http://xlink.rsc.org/?DOI=C6NJ01753C
DOI: 10.1039/C6NJ01753C |
| [62] |
Nie H, Li M J, Li Q S, Liang S J, Tan Y Y, Sheng L, Shi W, Zhang S X A. Chemistry of Materials, 2014,26(10):3104. http://dx.doi.org/10.1021/cm5003669
DOI: 10.1021/cm5003669 Two types of carbon dots (C dots) exhibiting respective excitation-independent blue emission and excitation-dependent full-color emissions have been synthesized via a mild one-pot process from chloroform and diethylamine. This new bottom-up synthetic strategy leads to highly stable crystalline C dots with tunable surface functionalities in high reproducibility. By detailed characterization and comparison of the two types of C dots, it is proved concretely that the surface functional groups, such as C=O and C=N, can efficiently introduce new energy levels for electron transitions and result in the continuously adjustable full-color emissions. A simplified energy level and electron transition diagram has been proposed to help understand how surface functional groups affect the emission properties. By taking advantage of the unique excitation-dependent full-color emissions, various new applications can be anticipated. Here, as an example, a ratiometric pH sensor using two emission wavelengths of the C dots as independent references has been constructed to improve the reliability and accuracy, and the pH sensor is applied to the measurement of intracellular pH values and cancer diagnosis. |
| [63] |
Sun M Y, Qu S N, Hao Z D, Ji W Y, Jing P T, Zhang H, Zhang L G, Zhao J L, Shen D Z. Nanoscale, 2014,6(21):13076. http://dx.doi.org/10.1039/c4nr04034a
DOI: 10.1039/c4nr04034a A new type of environmentally friendly phosphor based on carbon nanodots (CDs) has been developed through the dispersion of CDs by integrating the CDs with starch particles. The starch particles contain large numbers of hydroxyl groups around the surfaces, which can effectively absorb the CDs, whose surfaces are functionalized by lots of carboxyl and amide groups, through hydrogen bonding. Effective dispersion of CDs on the surfaces of starch particles can suppress the non-radiative decay processes and photoluminescence (PL) quenching induced by aggregation of CDs. The starch matrix neither competes for absorbing excitation light nor absorbs the emissions of CDs, which leads to efficient PL emitting. As a result, the starch/CD phosphors with a quantum yield of similar to 50% were obtained. The starch/CD phosphors show great potential in phosphor-based light emitting diodes, temperature sensors, and patterning. |
| [64] |
Zhou D, Zhai Y C, Qu S N, Li D, Jing P T, Ji W Y, Shen D Z, Rogach A L. Small, 2017,13(6):1602055. http://doi.wiley.com/10.1002/smll.v13.6
DOI: 10.1002/smll.v13.6 |
| [65] |
Liu K K, Li X M, Cheng S B, Zhou R, Liang Y C, Dong L, Shan C X, Zeng H B, Shen D Z. Nanoscale, 2018,10(15):7155. http://xlink.rsc.org/?DOI=C8NR01209A
DOI: 10.1039/C8NR01209A |
| [66] |
Kim T H, Wang F, McCormick P, Wang L Z, Brown C, Li Q. Journal of Luminescence, 2014,154:1. http://dx.doi.org/10.1016/j.jlumin.2014.04.002
DOI: 10.1016/j.jlumin.2014.04.002 UV and thermal stable, photoluminescent carbon dots (CDs) prepared by embedding CDs in ionic salt crystals such as NaCl, KCL, KBr are demonstrated. The salt crystal embedding matrix does not interfere with CDs strong emission, and provides effective protection to CDs from the environment. The degradation of 20% of the initial luminescence intensity of salt-encapsulated CDs (S-CDs) is 15 times slower under UV and 6 times slower under heat compared to that of CDs in silica matrix. We also demonstrate that the S-CDs can be applied as a color-converting phosphor for typical GaN UV light emitting diodes (LEDs) with significant improvements in stability as well as processability. (C) 2014 Elsevier B.V. |
| [67] |
Zhai Y C, Zhou D, Jing P T, Li D, Zeng H B, Qu S N. Journal of Colloid and Interface Science, 2017,497:165. https://linkinghub.elsevier.com/retrieve/pii/S0021979717302503
|
| [68] |
Wang Y, Kalytchuk S, Wang L Y, Zhovtiuk O, Cepe K, Zboril R, Rogach A L. Chemical Communication, 2015,51(14):2950. http://xlink.rsc.org/?DOI=C4CC09589H
DOI: 10.1039/C4CC09589H |
| [69] |
Green D C, Holden M A, Levenstein M A, Zhang S H, Johnson B R G, Gala de Pablo J, Ward A, Botchway S W, Meldrum F C. Nature Communications, 2019,10(1):206. https://doi.org/10.1038/s41467-018-08214-6
|
| [70] |
Wang J L, Zhang F, Wang Y L, Yang Y Z, Liu X G. Carbon, 2018,126:426. https://linkinghub.elsevier.com/retrieve/pii/S0008622317310424
|
| [71] |
Wang A W, Hou Y L, Kang F W, Lyu F C, Xiong Y, Chen W C, Lee C S, Xu Z T, Rogach A L, Lu J, Li Y Y. Journal of Materials Chemistry C, 2019,7(8):2207. http://xlink.rsc.org/?DOI=C8TC04171G
DOI: 10.1039/C8TC04171G |
| [72] |
Zhang Q H, Tian Y, Wang C F, Chen S. RSC Advances, 2016,6(53):47616. http://xlink.rsc.org/?DOI=C6RA05689J
DOI: 10.1039/C6RA05689J |
| [73] |
Hu S L, Ding Y L, Chang Q, Trinchi A, Lin K, Yang J L, Liu J. Nanoscale, 2015,7(10):4372. http://xlink.rsc.org/?DOI=C4NR07119K
DOI: 10.1039/C4NR07119K |
| [74] |
Chang Q, Yang S S, Xue C R, Li N, Wang Y Z, Li Y, Wang H Q, Yang J L, Hu S L. Nanoscale, 2019,11(15):7247. http://xlink.rsc.org/?DOI=C9NR01224A
DOI: 10.1039/C9NR01224A |
| [75] |
Wang D, Liu J G, Chen J F, Dai L M. Advanced Materials Interfaces, 2016,3(1), 1500439. http://doi.wiley.com/10.1002/admi.201500439
|
| [76] |
Zhou Z J, Tian P F, Liu X Y, Mei S L, Zhou D, Li D, Jing P T, Zhang W L, Guo R Q, Qu S N, Rogach A L. Advanced Science, 2018,5(8):1800369. http://doi.wiley.com/10.1002/advs.v5.8
DOI: 10.1002/advs.v5.8 |
| [77] |
Li D, Han D, Qu S N, Liu L, Jing P T, Zhou D, Ji W Y, Wang X Y, Zhang T F, Shen D Z. Light: Science & Applications, 2016,5(7):e16120.
|
| [78] |
Chen Y H, Zheng M T, Xiao Y, Dong H W, Zhang H R, Zhuang J L, Hu H, Lei B F, Liu Y L. Advanced Materials, 2015,28(2):312. http://doi.wiley.com/10.1002/adma.201503380
|
| [79] |
Jiang B P, Yu Y X, Guo X L, Ding Z Y, Zhou B, Liang H, Shen X C. Carbon, 2017,128:12. https://linkinghub.elsevier.com/retrieve/pii/S0008622317311843
|
| [80] |
Shen C L, Zang J H, Lou Q, Su L X, Li Z, Liu Z Y, Dong L, Shan C X. Carbon, 2018,136:359. https://linkinghub.elsevier.com/retrieve/pii/S0008622318304743
|
| [81] |
Liu J C, Wang N, Yu Y, Yan Y, Zhang H Y, Li J Y, Yu J H. Science Advances, 2017,3(5):e1603171. https://advances.sciencemag.org/lookup/doi/10.1126/sciadv.1603171
|
| [82] |
Yang H Y, Liu Y L, Guo Z Y, Lei B F, Zhuang J L, Zhang X J, Liu Z M, Hu C F. Nature Communication, 2019,10(1):1789. https://doi.org/10.1038/s41467-019-09830-6
|
| [83] |
Zhou D, Jing P T, Wang Y, Zhai Y C, Li D, Xiong Y, Baranov A V, Qu S N, Rogach A L. Nanoscale Horizons, 2019, 4, ( 2):388. http://xlink.rsc.org/?DOI=C8NH00247A
DOI: 10.1039/C8NH00247A |
| [84] |
Zhang Y Q, Zhuo P, Yin H, Fan Y, Zhang J H, Liu X Y, Chen Z Q. ACS Applied Materials & Interfaces, 2019,11(27):24395. https://pubs.acs.org/doi/10.1021/acsami.9b04600
|
| [85] |
Wang H J, Yu T T, Chen H L, Nan W B, Xie L Q, Zhang Q Q. Dyes and Pigments, 2018,159:245 https://linkinghub.elsevier.com/retrieve/pii/S0143720818309987
|
| [86] |
Zhang Y Q, Li C F, FanY, Wang C B, Yang R F, Liu X Y, Zhou L. Nanoscale, 2016,8(47):19744. http://xlink.rsc.org/?DOI=C6NR06553H
DOI: 10.1039/C6NR06553H |
| [87] |
Yeh H C, Wu W C, Chen C T. Chemical Communications, 2003, ( 3):404.
|
| [88] |
Hong Y N, Lam J W Y, Tang B Z. Chemical Communications, 2009, ( 29):4332.
|
| [89] |
Qu S N, Liu X Y, Guo X Y, Chu M H, Zhang L G, Shen D Z. Advanced Functional Materials, 2014,24(18):2689. http://onlinelibrary.wiley.com/doi/10.1002/adfm.201303352/abstract
In this work, the optical properties of carbon nanoparticles (CNPs) can be modulated by the dopant-N atom and sp(2) C-contents. CNPs prepared with the low urea mass ratio of 0.2:1 (CNP1) exhibit blue emission (maximum PL quantum yield: 15%). Increasing sp(2) C- and dopant-N atom contents, as determined in CNPs prepared with high urea mass ratio of 2:1 (CNP2), lead to green emission (maximum PL quantum yield up to 36% in ethanol aqueous solution). Amplified spontaneous emission (ASE) can be observed only in CNP2 ethanol aqueous solution. Green lasing emission is achieved from CNP2 ethanol aqueous solution in a linear long Fabry-Perot cavity, indicating the potential of CNP2 as a gain medium for lasing. CNP2 shows superior photostability compared with C545T dye. The green emission from CNP2 is speculated to arise from electron-hole recombination (intrinsic state emission). The high PL quantum yield and small overlap between absorption and emissions of CNP2 ethanol aqueous solution are the key factors in realizing lasing emission. |
| [90] |
Dong Y Q, Pang H C, Yang H B, Guo C X, Shao J W, Chi Y W, Li C M, Yu T. Angew. Chem. Int. Ed., 2013,52(30):7800. http://doi.wiley.com/10.1002/anie.v52.30
DOI: 10.1002/anie.v52.30 |
| [91] |
Hu S L, Tian R X, Dong Y G, Yang J L, Liu J, Chang Q. Nanoscale, 2013,5(23):11665. http://dx.doi.org/10.1039/c3nr03893a
DOI: 10.1039/c3nr03893a To demonstrate the effects of surface atoms on photoluminescence (PL) and photocatalytic activities of luminescent carbon dots (CDs), we design and tailor the surface groups of CDs with heteroatoms by a facile and effective approach. The coexistence of O and N radicals in CDs results in strong PL while CDs containing O and Cl radicals show high photocatalytic activity. This is attributed to the different degrees and directions of energy band bending from inner to surface induced by O, N, and Cl radicals at the surface of CDs. The coexistence of both upward and downward band bending that are caused by the O and Cl radicals, respectively, in CDs is similar to an internal electronic field that facilitates the separation of electron-hole pairs and carrier migration, leading to high photocatalytic activity. These results may also be used for designing and tailoring optical-electronic properties of carbon nanostructures. |
| [92] |
Tian R X, Hu S L, Wu L L, Chang Q, Yang J L, Liu J. Applied Surface Science, 2014,301:156. http://dx.doi.org/10.1016/j.apsusc.2014.02.028
DOI: 10.1016/j.apsusc.2014.02.028 A facile and green method to tailor surface groups of carbon quantum dots (CQDs) is developed by hydrothermal treatment in an autoclave. The photoluminescence (PL) behaviors of CQDs depend on the types of surface groups. Highly efficient photoluminescence is obtained through amino-hydrothermal treatment of the CQDs reduced by NaBH4. The effects of surface groups on PL behavior are attributed to the degrees of energy band bending induced by surface groups. (C) 2014 Elsevier B.V. |
| [93] |
Zhu S J, Meng Q N, Wang L, Zhang J H, Song Y B, Jin H, Zhang K, Sun H C, Wang H Y, Yang B. Angew. Chem. Int. Ed., 2013,52(14):3953. http://doi.wiley.com/10.1002/anie.v52.14
DOI: 10.1002/anie.v52.14 |
/
| 〈 |
|
〉 |