

文章编号: 2020020308
文献标识码: A
人参皂苷类化合物样品前处理及分析检测
收稿日期:2019-08-13
要求修回日期:2019-09-13
网络出版日期:2019-11-18
基金资助
烟台市重点研发计划(2018ZHGY085)
药学国家级实验教学示范中心(烟台大学)()
版权
Sample Pretreatment, Analysis and Detection of Ginsenosides
Received:13 Aug. 2019
rev-requestrev-request:13 Sept. 2019
Online:18 Nov. 2019
Fund
Key Research Project of Yantai City(2018ZHGY085)
National Demonstration Center for Experimental Pharmacy Education(Yantai University)()
Copyright
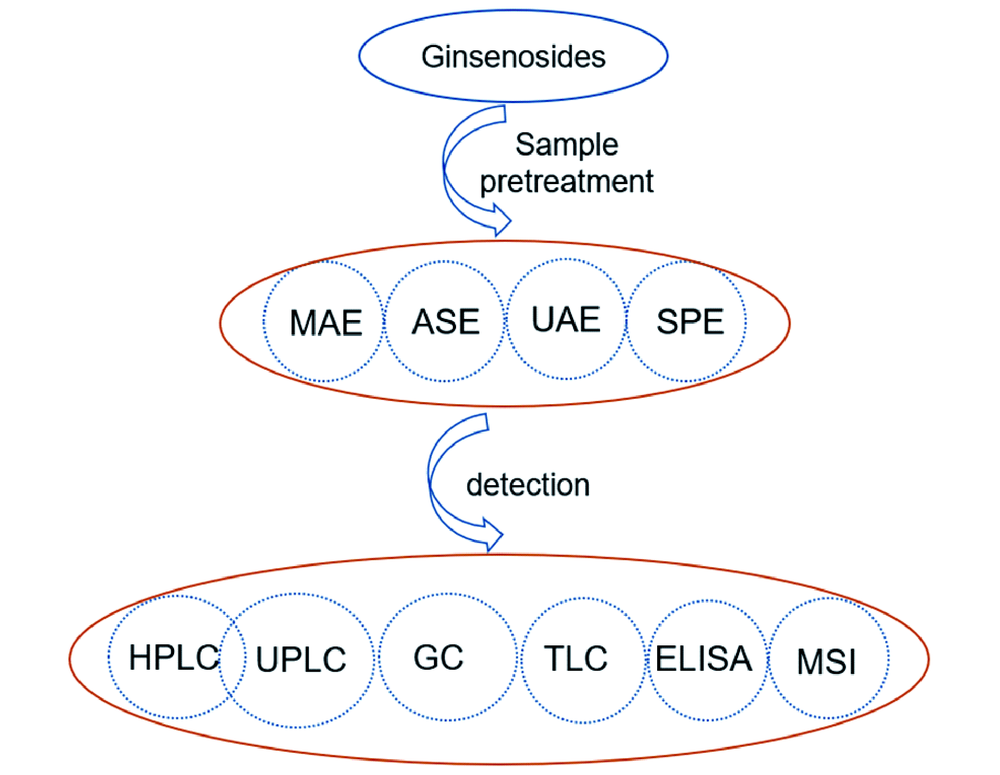
人参皂苷类化合物是参属类植物中的一类重要活性成分,主要包括原人参二醇、原人参三醇、齐墩果酸型、奥克梯隆型四类。最近,已发现的人参皂苷类化合物化学结构多达620余种,它们具有相似的化学结构,但药理活性具有明显差异。该类化合物所在基质复杂多样,选择简便高效的样品前处理方法及检测技术对于有效检测样品中人参皂苷含量至关重要。本文综述了测定各类样品中多种人参皂苷含量的样品前处理技术(溶剂提取、固相萃取等)及常用检测方法(高效液相色谱法、超高效液相色谱法、薄层色谱法、气相色谱法等),对各种方法的灵敏度及回收率等参数进行了总结,并评述了每种方法的优缺点及研究进展。
宋志花 , 李盛红 , 杨刚强 , 周娜 , 陈令新 . 人参皂苷类化合物样品前处理及分析检测[J]. 化学进展, 2020 , 32(2/3) : 239 -248 . DOI: 10.7536/PC190814
Zhihua Song , Shenghong Li , Gangqiang Yang , Na Zhou , Lingxin Chen . Sample Pretreatment, Analysis and Detection of Ginsenosides[J]. Progress in Chemistry, 2020 , 32(2/3) : 239 -248 . DOI: 10.7536/PC190814
Ginsenosides are an important kind of active ingredients in Panax genus and can be classified into four main types: the protopanaxadiols(PPD), protopanaxatriols(PPT), oleanolic acids(OA) and ocotillol type(OT). Recently, more than 620 kinds of these compounds have been isolated. Their chemical structures are similar but have quite different medicinal functions. It is of vital importance to develop simple and facile sample pretreatment methods and detection techniques to detect the content of ginsenosides in complex matrix. This review includes many kinds of sample pretreatment methods(such as liquid phase extraction, and solid phase extraction) and detection methods(such as high performance liquid chromatography, ultra-performance liquid chromatography, thin layer chromatography, and gas chromatography), etc., summarizes the sensitivity and recovery of various methods, and reviews the advantages and disadvantages of each method and its research progress.
Key words: ginsenosides ; sample pretreatment ; analysis methods
表1 四类常见人参皂苷化合物:(a)20(s)-原人参二醇、(b)20(s)-原人参三醇、(c)齐墩果酸型、(d)24(R)-奥克梯隆型[13]Table 1 Four types of common ginsenosides:(a) 20(s)-PPD,(b) 20(s)-PPT,(c) OA,(d) 24(R)-OT[13]. Copyright 2000 American Chemical Society |
(a) 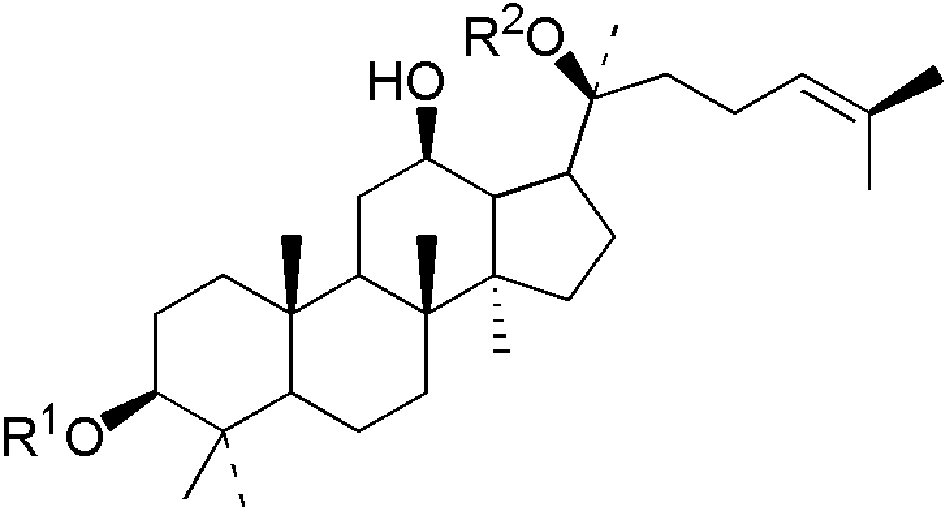 | ginsenoside | R1 | R2 | formula | M.W. | |
|---|---|---|---|---|---|---|
| Rb1 | -glc[2→1]glc | -glc[6→1]glc | C54H92O23 | 1108 | ||
| Rb2 | -glc[2→1]glc | -glc[6→1]ara(p) | C53H90O22 | 1078 | ||
| Rc | -glc[2→1]glc | -glc[6→1]ara(f) | C53H90O22 | 1078 | ||
| Rd | -glc[2→1]glc | -glc | C48H82O18 | 946 | ||
(b) 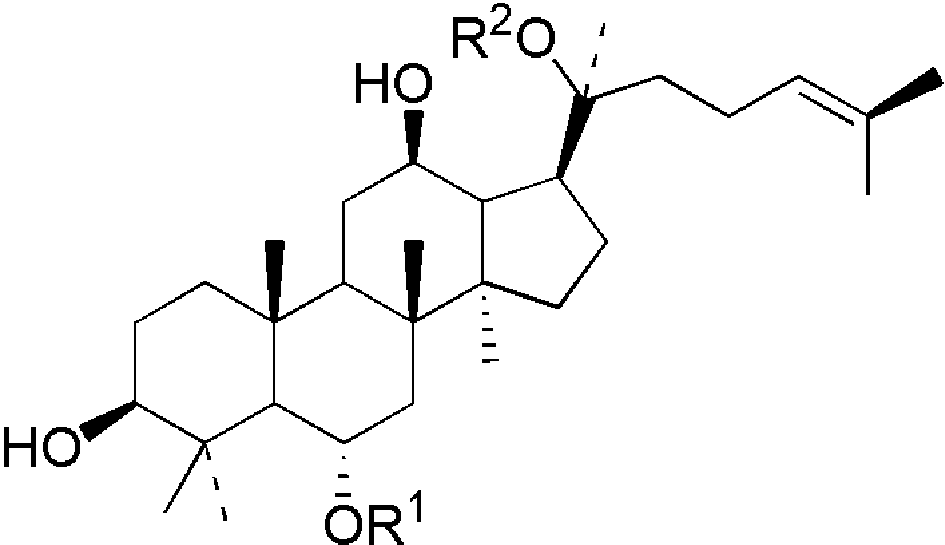 | ginsenoside | R1 | R2 | formula | M.W. | |
| Rg1 | -glc | -glc | C42H72O14 | 800 | ||
| Re | -glc[2→1]rha | -glc | C48H82O18 | 946 | ||
| Rf | -glc[2→1]glc | -H | C42H72O14 | 800 | ||
(c) 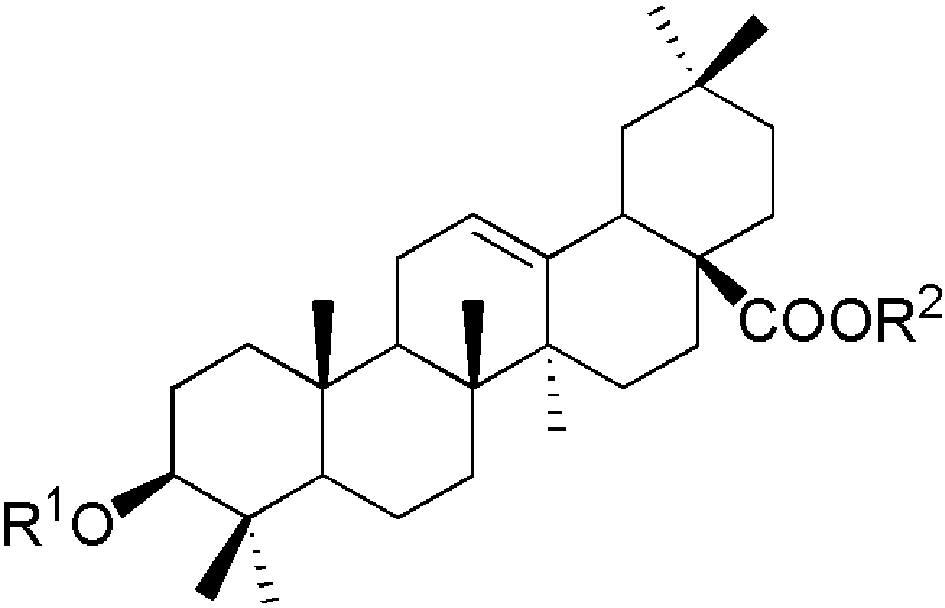 | ginsenoside | R1 | R2 | formula | M.W. | |
| Ro | -glcUA[2→1]glc | -glc | C48H76O19 | 956 | ||
(d) 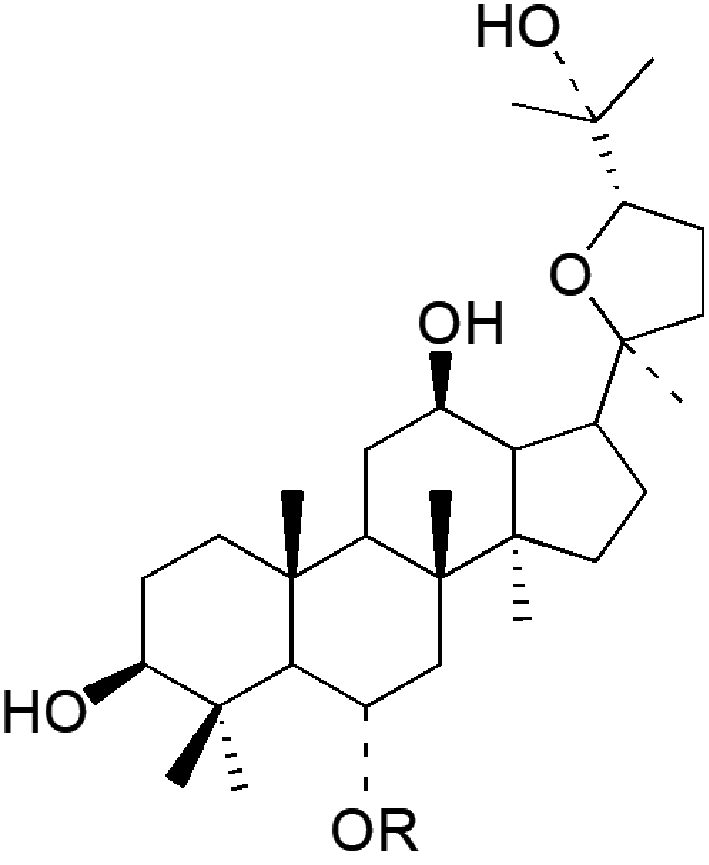 | pseudoginsenoside | R | formula | M.W. | ||
| F11 | -glc[2→1]rha | C42H72O14 | 800 |
Sugar units: glc: β-D-glucose; ara(p): α-L-arabinofuranose; rha: α-L-rhamnose; glcUA: β-D-glucuronide. |
表2 人参皂苷类化合物的样品前处理方法Table 2 Sample pretreatment methods for ginsenosides |
| Sample pretreatment methods | Principles | Advantages | Disadvantages | |
|---|---|---|---|---|
| Solvent extraction method | Microwave assisted extraction(MAE) | Cell walls of the Chinese traditional medicine are disrupted by the microwave with energy of 300 MHz~300 GHz, and then the extracting rate is increased. | Convenient operation, short extraction time, high extraction yield, less solvent consumption, and low cost[24, 25]. | Some heat-sensitive compounds are easy to be destroyed. |
| Ultrasonic assisted extraction(UAE) | The disruption of cell walls, reduction of particle-size and enhancing mass transfer of the cell contents are caused by cavitation bubble collapse, mechanical and thermal effects. | Highly efficient, low consumption of solvent, fast(tens minutes), mild conditions(20~30 ℃), simple and low cost[26]. | Low degree of automation and difficult to be used on-line. | |
| Accelerated solvent extraction(ASE) | ASE is carried out in closed container with a high pressure and a temperature above the boiling point of organic solvent. The extraction rate increases with the increase of pressure and temperature. | Fast(a few minutes to ten minutes), save solvent, realize automation easily. | Some heat-sensitive compounds are easy to be destroyed; the instruments are expensive; and operation skills are hard to master. | |
| Sub-and supercritical fluid extraction | The extraction process proceeds by using sub-and supercritical fluids near the critical point of temperature and pressure with the good properties of high-density, low-viscosity, and high-permeability. | Non-toxic and safe, no organic solvent residual, environment friendly, low energy cost [27]. | The instrument is expensive due to high pressure resistant. | |
| Enzymatic dissociation method | Active ingredients can beextracted from plant tissues by using suitable enzymes(cellulase, amylase, etc.) under mild conditions[28]. Furthermore, the fragmentation of cell walls will be accelerated with the increase of the pressure(100~600 MPa) [29]. | Efficient and has great potential in extraction of active ingredients from traditional Chinese medicine. | Susceptible to external conditions. | |
| Solid phase extraction (SPE) | The ginsenoside compounds and impurities are separated by adsorbents according to the difference in adsorption. | Simple, low cost and wide range ofapplication. | Large amount of organic reagents consumption, only suitable for pretreatment of small batches of samples. | |
表3 液相色谱法检测人参皂苷类化合物Table 3 The application of liquid chromatography for analysis of active compounds in Chinese herbs |
| Analyte | Matrix | Sample pretreatment | Column and temperature | Mobile phases and detection | Recovery(%) | LOD | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Re, Rh1, Rg2, Rg1, Rf | White ginseng, red ginseng, American ginseng, and ginseng. | Extracted by methanol | Shiseido UG 80 Capcell Pak NH2 column (250 × 4.6 mm i.d., 5 μm), 25 ℃ | 0~3 min, 89% A; 3~25 min, 89%→84% A; 25~30 min, 84%→82% A; 30~35 min, 82%→76% A; 35~40 min,89% A. A:acetonitrile; B water. UV:203 nm | 95.31%~103.85% | 0.0047 ~ 0.225 (mg·L-1) | 12 | ||||||
| 20 (S)-Rh1, 20 (R)-Rh1, Rg6, F4, Rk3, 20(S)-Rg3, 20(R)-Rg3, Rk1, Rg5 | Roots of P. quinquefolius L. | Reflux extraction by water and methanol | Acchrom Technologies ODS-C18 type column(250 ×4.6 mm i.d., 5 μm), 30 ℃. | 0~10 min, 33% B; 10~15 min, 33%→40% B; 15~40 min, 40%→60% B; 40~70 min, 60% B. A: water; B: acetonitrile. HPLC-UV: 203 nm. HPLC-ESI-MS | 97.97%~103.24% | 0.18~ 0.45 (μg·mL-1) | 24 | ||||||
| ginsenosides Rb1, Rc, Rb2, Rb3; notoginsenosides Fc, Fe, Fd | Panax notoginseng leaves | Rxtracted by methanol | Agilent Zorbax ODS C8 column(250 × 4.6 mm i.d.,5 μm), 35 ℃ | 0~5 min, 15%→30% B; 5~15 min, 30%→32% B; 15~35 min, 32%→32% B; 35~45 min, 32%→45% B; 45~60 min, 45%→50% B. A: water; B: acetonitrile. HPLC-UV: 203 nm | 98.7%~106.1% | 98 (ng) | 40 | ||||||
| Ginsenoside Rg1, Ginsenoside Rb1, Ginsenoside Rc, Ginsenoside Rd, Ginsenoside Re, Ginsenoside Rf, Ginsenoside Rg3, Ginsenoside Rh1, Ginsenoside Rb2, Ginsenoside Rb3 | Ginsenosides in chronic heart failure(CHF) rats | Solid-phase extraction(SPE) | ACQUITY UPLC HSS T3 column(100 × 2.1 mm i.d., 1.8 μm), 40 ℃ | 0~1 min, 30%→35% B; 1.0~5.0 min, 35%→38% B; 5.0~5.5 min, 38%→45% B; 5.5~6.0 min, 45%→80% B; 6.0~7.0 min, 80%→90% B; 7.0~7.5 min, 90%→30% B; 7.5~8.0 min, 30%→30% B. A: water with 0.1% formic acid; B: acetonitrile. UFLC-MS/MS | 60%~105% | - | 42 | ||||||
| Ginsenoside Rg1, Ginsenoside Re, Ginsenoside Rb1 | Panax quinquefolii Radix | Reflux extraction by water-saturated n-butanol | Venusil XBP C18 column (250 × 4.6 mm i.d., 5 μm), 30 ℃. | 0.01~25 min, 19%→20% A; 25.01~60 min, 20%→40% A; 60.01~80 min, 40.1%→100% A. A: acetonitrile; B: 0.1% phosphoric acid solution. UV: 203 nm | 88.61%~94.29% | 0.066~ 0.400 (μg·mL-1) | 55 | ||||||
| notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, astragaloside Ⅳ, ginsenoside Rd | Qishen Yiqi Dripping Pills(QYDP) | liquid-liquid Extraction using water- saturated n-butanol | Acquity UPLC HSS T3 column (100 × 2.1 mm i.d., 5 μm), 30 ℃ | 0~1 min, 83% A; 1~14 min, 83%→56%; 14~15 min, 56% A. A: water containing 0.1% formic acid; B: acetonitrile. UPLC-ELSD | 96.87%~99.97% | 2.36~ 7.68 (μg·mL-1) | 57 | ||||||
| Analyte | Matrix | Sample pretreatment | Column and temperature | Mobile phases and detection | Recovery(%) | LOD | Ref | ||||||
| G-Ra1, G-Ra2, G-Rb1, G-Rb2, G-Rb3, G-Rc, G-Rd, G-Re, G-Re4, G-Rf, G-Rg1, G-Rg2, G-Ro, G-Rs2, G-RoMe, 20-Glc- G-Rf, Ma-G-Rb2, NG-R1, NG-R2 | Roots and rhizomes of Panax ginseng samples. | Extracted by 70% aqueous MeOH solutions | Diamonsil ODS C18 column (250 × 4.6 mm i.d., 5 μm), room temperature | 0~20 min, 10%→20% A; 20~30 min, 20%→22% A; 30~40 min, 22%→31%A; 40~75 min, 31%→33% A; 75~80 min, 33%→40% A; 80~90 min, 40%→50% A; 90~100 min, 50%→60% A; 100~110 min, 60%→70% A. Flow rate: 0~32 min, 0.8 mL/min; 32.1~110 min, 0.5 mL/min. A: MeCN; B: MeCN: H2O: 0.1% formic acid aqueous solution(5∶90∶8; v/v/v) HPLC-ESI-MS | 94.87%~102.45% | 0.159~ 9.052 (ng) | 58 | ||||||
| N-R1, G-Rg1, G-Re, G-Rf, G-F3, G-Rg2, G-Rh1, G-Rb1, G-Ro, G-Rc, G-Rb2, G-Rb3, CS-IV, CS-Iva, G-Rd, G-Rg3 | Panax japonicas(PJ), Panax japonicus var. major(PM), and Panax zingiberensis (PZ) | Extracted by 60% aqueous methanol solutions | Waters C18 column(150×3.9 mm i.d., 4.6 μm), room temperature | 0~3 min, 20%→23% A; 3~8 min, 30%→35% A; 8~15 min, 35% A; 15~20 min, 35%→60% A; 20~22 min, 60%→80% A; 22~24 min, 80%→95% A; 24~25 min, 95%→20% A. A: acetonitrile; B: 0.05% formic acid aqueous solution. HPLC-ESI-MS/MS | 99.25%~104.10% | 0.13~ 2.22 (ng·mL-1) | 59 | ||||||
| ginsenosides Rg1, 20(S)-Rg2, Re, 20(S)-Rh, Rb1, Rb2, Rd | Tissue extracts from the root and rhizome of Panax ginseng C.A. Mey. | Extracted by methanol | Waters C18 column(100 mm ×2.1 mm i.d., 1.7 μm), room temperature ~20 ℃. | 0~3 min, 10%→20% B; 3~25 min, 20%→38% B; 25~30 min, 38%→85% B; 30~30.1 min, 85%→100% B; 30.1~32 min, 100% B; 32~32.1 min 100%→10% B. A: formic acid aqueous solution; B: acetonitrile containing 0.1% formic acid. UPLC-QTOF-MS | - | 6.08~ 108.72 (ng·mL-1) | 60 | ||||||
| Rg1 and its metabolites | Sprague- Dawley rat bile, urine, and feces | Extracted by methanol | Shim-Pack XR-ODS Ⅱ(75 × 2 mm, 2.3 μm) column, 40 ℃. | 0~7 min, 22%→80% B; 7~7.01 min, 80%→22%; 7.01~10 min, 22%→22% B. A: 0.05% formic acid aqueous solution; B: 0.05% formic acid in acetonitrile. HPLC-MS/MS | Rg1, ginsenoside Rh1(Rh1), and protopanaxatriol(Ppt) in bile, urine, and feces ≥70%. The fecal excretion recoveries of Rg1, Rh1, and Ppt, 22.19%~ 40.11%. Rg1 in bile, 6.88%; Rh1 and Rg1in Urinary excretion 0.04%~0.09%. | - | 61 | ||||||
| Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rd | Kang’ai injection | Aqueous two- phase system based Deep eutectic solvent and K2HPO4 solution | Agilent Zorbax SB-C18 column(250×4.6 mm i.d., 5 μm), 30 ℃ | 0~34 min, 19.2% A; 34~35 min, 19.2%→28.0% A; 35~48 min, 28.0% A; 48~56 min, 28.5% A; 56~72 min, 36.0% A. A: acetonitrile; B: 0.1% phosphoric acid aqueous solution. HPLC-DAD: 203 nm | 92.7%~110.8% | 0.3~1.5 (μg·mL-1) | 62 | ||||||
| [1] |
Gurung B, Bhardwaj P K, Rai A K, Sahoo D . Nat. Prod. Res., 2018,32:234. https://www.ncbi.nlm.nih.gov/pubmed/28649854
DOI: 10.1080/14786419.2017.1343322 PMID: 28649854 This study compared eight major ginsenosides (Rg1, Rg2, Rf, Re, Rd, Rc, Rb1 and Rb2) between Panax sokpayensis and Panax bipinnatifidus collected from Sikkim Himalaya, India. High-performance liquid chromatographic analysis revealed that all major ginsenosides were present in the rhizomes of P. sokpayensis except ginsenoside Rc, whereas ginsenoside Rf, Rc and Rb2 were not detected in P. bipinnatifidus. |
| [2] |
Gao Y L, Wang T, Wang G F, Li G S, Sun C F, Jiang Z M, Yang J R, Li Y S, You Y L, Wu X R, Sun L Q, Wang H B, Li C M, Tian J W, Zhu J, Wang K Z . Cho S. Food Chem. Toxicol., 2019,131:110578. https://www.ncbi.nlm.nih.gov/pubmed/31201900
DOI: 10.1016/j.fct.2019.110578 PMID: 31201900 Ginsenoside compound K (CK) is a hydrolysate of ginsenosides in the soil bacteria. This study evaluated the toxicity of CK as acute and the 26-week repeated-dose. The results of acute toxicity show that CK administered orally to rats and mice did not cause mortality or toxicity at the maximum dosage of 8 g/kg and 10 g/kg, respectively. In the toxicity study for 26-week, rats were administered with CK at doses of 13, 40, or 120 mg/kg, and were observed for 26 weeks and recovery periods of four weeks. Under the conditions, asthenia, hypoactivity, loss of fur and body weight reduction were transiently noticed in males of 120 mg/kg group. Hepatotoxicity and nephrotoxicity also were evident including the elevation of liver and kidney relative weight, along with focal liver necrosis as well as the increase in plasma enzymes (ALT and ALP) in male rats receiving CK (120 mg/kg), but this toxicity might be reversible. For 13 and 40 mg/kg CK groups, there was no significant variation in food habits, clinical signs, urine analysis, body weight, biochemical and hematological values, organ coefficient and histopathology examination. The NOAEL for male and female rats were observed to be 40 and 120 mg/kg, respectively. |
| [3] |
Wang W Y, Ni Y Y, Wang L, Che X, Liu W H, Meng Q G . Xenobiotica, 2015,45:385. https://www.ncbi.nlm.nih.gov/pubmed/25430797
DOI: 10.3109/00498254.2014.986562 PMID: 25430797 1. In this study, the oxidative metabolites of 20(S)-protopanaxatriol (PPT) were identified in human liver microsomes (HLMs) and in rats using liquid chromatography-electrospray ionization tandem mass spectrometry. 2. Twelve oxidative metabolites were found in HLM, eight of which have not been previously reported. Twenty-four oxidative metabolites were found in rat feces after oral administration and 20 of these, including six found in HLM, were first reported. The results indicated PPT was more extensively metabolized in rats than in HLM. C20-24 epoxides, a pair of epimers (namely, M1-1 and M1-2) were the major metabolites, and other metabolites were derived from their further metabolism. 3. Enzyme kinetics experiments showed that the apparent formation Vmax of M1-1 was 10.4 folds and 2.4 folds higher than that of M1-2 in HLM and in rat liver microsomes (RLMs), respectively. The depletion rate of M1-2 was 11.0 folds faster than M1-1 in HLM, and was similar in RLM. Hence, the remarkable species differences of PPT metabolism mainly resulted from the stereoselective formation and further metabolic elimination of M1-1 and M1-2. 4. Chemical inhibition study and recombinant human P450 isoforms analysis showed that CYP3A4 was the predominant isoform involved in the oxidative metabolism of M1-1 and M1-2. |
| [4] |
Ren Q W, Yang G Q, Guo M Q, Guo J W, Li Y, Lu J, Yang Q, Tang H H, Li Y, Fang X J, Sun Y X, Qi J G, Tian J W, Wang H B . Eur. J. Med. Chem., 2019,161:118. https://www.ncbi.nlm.nih.gov/pubmed/30347326
DOI: 10.1016/j.ejmech.2018.10.038 PMID: 30347326 Multidrug resistance (MDR) is a major cause of failure in cancer treatment, in which the overexpression of P-glycoprotein (Pgp) plays a crucial role. Herein, a novel class of ocotillol-type amide derivatives has been designed, synthesized, and evaluated for their ability to reverse MDR. The structure-activity relationship of the reversal activity was analyzed. Ten compounds showed promising chemo-reversal ability, among which the 24R-ocotillol-type amide derivative 6c with an N-Boc-hexanoyl unit exhibited the most potency in reversing paclitaxel resistance in KBV cells. Compound 6c could inhibit Pgp-mediated rhodamine123 efflux function via stimulating Pgp-ATPase activity and exhibited high binding affinity with Pgp in molecular docking studies. Importantly, compound 6c enhanced the efficacy of paclitaxel against KBV cancer cell-derived xenograft tumors in nude mice after oral administration. These results indicate that ocotillol-type amide derivatives are promising lead compounds for overcoming MDR in cancer. |
| [5] |
Xu X F, Gao Y, Xu S Y, Liu H, Xue X, Zhang Y, Zhang H, Liu M N, Xiong H, Lin R C, Li X R . J. Ginseng Res., 2018,42:277. https://www.ncbi.nlm.nih.gov/pubmed/29983609
DOI: 10.1016/j.jgr.2017.02.003 PMID: 29983609 Temperature is an essential condition in red ginseng processing. The pharmacological activities of red ginseng under different steam temperatures are significantly different. |
| [6] |
Liu Z Y, Zhang H Y, Bi Y, Liu X X, Lu J, Zhang X C, Xu J Y, Wang C Z, Yuan C S . Nat. Prod. Res., 2017,31:1523. https://www.ncbi.nlm.nih.gov/pubmed/28107791
DOI: 10.1080/14786419.2017.1280488 PMID: 28107791 To explore the antitumour mechanism of 20(S)-protopanaxadiol (PPD) while maintaining its uncovered pharmacological active site 3-hydroxyl, 28-hydroxy protopanaxadiol (17), a small molecular probe template of PPD was first designed and synthesised based on the Baldwin's reaction. Thus, 28-hydroxyl of 17 was built successfully as a derivatized site of molecular probe's functional and report groups. The important intermediates and final product were confirmed by ESI-MS and nuclear magnetic resonance spectra with good yield. These studies provided a valuable basis for probe research of PPD. |
| [7] |
Liu F, Ma N, Xia F B, Li P, He C W, Wu Z Q, Wan J B . J. Ginseng Res., 2019,43:105. https://www.ncbi.nlm.nih.gov/pubmed/30662299
DOI: 10.1016/j.jgr.2017.09.003 PMID: 30662299 Ginsenosides with less sugar moieties may exhibit the better adsorptive capacity and more pharmacological activities. |
| [8] |
Song Y Q, Zhao F, Zhang L M, Du Y, Wang T, Fu F H . Fitoterapia, 2013,91:173. https://www.ncbi.nlm.nih.gov/pubmed/24035860
DOI: 10.1016/j.fitote.2013.09.001 PMID: 24035860 Glucocorticoids (GCs) are usually used to treat inflammatory diseases. However, they cause severe and irreversible side effects, which limit the use of these compounds. Ginsenoside Rg1 had been demonstrated to possess anti-inflammatory and anti-cancer effects. The present study was designed to investigate whether Rg1 exhibits synergistic anti-inflammatory effects when combined with glucocorticoids. After stimulated by lipopolysaccharide (LPS), murine macrophagic RAW264.7 cells were treated with Rg1, corticosterone (Cort) or Rg1 and Cort. Then nitric oxide (NO), tumor necrosis factor-α (TNF-α) and glucocorticoid receptor (GR) expression were measured. The results showed that Rg1 or Cort could reduce the production of NO and TNF-α, and Rg1 dose-dependently up-regulated GR expression, while Cort dose-dependently down-regulated GR expression. The combination of low concentrations of Rg1 with Cort, which alone could not markedly inhibit the release of inflammatory factors, inhibited the secretion of NO and TNF-α in LPS-stimulated RAW264.7 macrophage cells, and up-regulated the expression of GR. The findings suggested Rg1 can synergize with glucocorticoid to enhance its anti-inflammatory effect. |
| [9] |
杨刚强(Yang G Q), 李阳(Li Y), 杨青(Yang Q), 岳馨(Yue X), 姚雷(Yao L), 姜永涛(Jiang Y T) . 有机化学 (Chinese Journal of Organic Chemistry), 2017,37:1530.
|
| [10] |
An K S, Choi Y O, Lee S M, Ryu H Y, Kang S J, Yeon Y, Kim Y R, Lee J G, Kim C J, Lee Y J, Kang B J, Choi J E, Song K S . Nutrients, 2019,11:1120.
|
| [11] |
Zhang J Q, Zhang Q, Xu Y R, Li H X, Zhao F L, Wang C M, Liu Z, Liu P, Liu Y N, Meng Q G, Zhao F . Planta Med., 2019,85:292. https://www.ncbi.nlm.nih.gov/pubmed/30380571
DOI: 10.1055/a-0770-0994 PMID: 30380571 in vitro anti-inflammatory activity of C20 epimeric ocotillol-type triterpenes (2, 3, 9: , and 10: ) and protopanaxadiol [20(S/R)-protopanaxadiol] were investigated. Epimers 2: and 3: were prepared starting from 20(S)-protopanaxadiol. Epimers 9: and 10: were synthesized from 20(R)-3-acetylprotopanaxadiol (7: ). The anti-inflammatory activity of 2, 3, 9, 10: , 20(S)-protopanaxadiol, and 20(R)-protopanaxadiol was evaluated in cultured mouse macrophage RAW 264.7 cells. The MTT assay was used to measure the cytotoxicity. RAW 264.7 cells were stimulated by lipopolysaccharide to release the inflammatory mediators nitric oxide, prostaglandin E2, TNF-α, and interleukin-6 and anti-inflammatory mediator interleukin-10. The effect of the compounds on the overproduction of nitric oxide, prostaglandin E2, TNF-α, interleukin-6, and interleukin-10 was determined using Griess and ELISA methods. The results demonstrated that the in vitro anti-inflammatory activities of C20 epimeric ocotillol-type triterpenes and protopanaxadiol were different. Both the 20S-epimers (2: and 3: ) and 20R-epimers (9: and 10: ) inhibited the release of inflammatory mediator nitric oxide, while mainly the 20S-epimers inhibited the release of inflammatory mediator prostaglandin E2, and the 20R-epimers inhibited the release of inflammatory cytokine TNF-α. Both the 20S-epimers [2, 3: , and 20(S)-protopanaxadiol] and 20R-epimers [9, 10: , and 20(R)-protopanaxadiol] inhibited the release of inflammatory cytokine interleukin-6, but mainly the 20S-epimers [2, 3: , and 20(S)-protopanaxadiol] increased the release of anti-inflammatory mediator interleukin-10.]]> |
| [12] |
Zhang L N, Wang S Y, Qu B Q, Chi H J, Quan Y L, Wu X H . J. Pharmaceut. Biomed., 2019,170:48.
|
| [13] |
Chan T W D, But P P H, Cheng S W, Kwok M Y I, Lau F W, Xu H X . Anal. Chem., 2000,72:1281. https://www.ncbi.nlm.nih.gov/pubmed/10740871
DOI: 10.1021/ac990819z PMID: 10740871 An LC/MS-based method is established for the differentiation and authentication of specimens and commercial samples of Panax ginseng (Oriental ginseng) and Panax quinquefolius (American ginseng). This method is based on the separation of ginsenosides present in the ginseng methanolic extracts using high-performance liquid chromatography (HPLC), followed by detection with electrospray mass spectrometry. Differentiation of ginsenosides is achieved through simultaneous detection of intact ginsenoside molecular ions and the ions of their characteristic thermal degradation products. An important parameter used for differentiating P. ginseng and P. quinquefolius is the presence of ginsenoside Rf and 24-(R)-pseudoginsenoside F11 in the RICs of Oriental and American ginsengs, respectively. It is important to stress that ginsenoside Rf and 24(R)-pseudoginsenoside F11, which possess the same molecular weight and were found to have similar retention times under most LC conditions, can be unambiguously distinguished in the present HPLC/MS method. The method developed is robust, reliable, reproducible, and highly sensitive down to the nanogram level. |
| [14] |
Chen W, Balan P, Popovich D. G ., J. Ginseng Res., 2019, DOI: 10.1016/j.jgr.2019.04.007.
|
| [15] |
Huang X, Liu Y, Zhang Y, Li S P, Yue H, Chen C B, Liu S Y . J. Ginseng Res., 2019,43:27. https://www.ncbi.nlm.nih.gov/pubmed/30662291
DOI: 10.1016/j.jgr.2017.08.001 PMID: 30662291 Panax quinquefolium L. under steaming were investigated, and the possible mechanisms were discussed.]]> |
| [16] |
Liu J, Xu Y R, Yang J J, Wang W Z, Zhang J Q, Zhang R M, Meng Q G . J. Ginseng Res., 2017,41:373. https://www.ncbi.nlm.nih.gov/pubmed/28701880
DOI: 10.1016/j.jgr.2017.01.001 PMID: 28701880 Panax quinquefolius L., Panax japonicus, Hana mina, and Vietnamese ginseng. In recent years, the semisynthesis of 20(S/R)-ocotillol-type saponins has been reported. The biological activities of ocotillol-type saponins include neuroprotective effect, antimyocardial ischemia, antiinflammatory, antibacterial, and antitumor activities. Owing to their chemical structure, pharmacological actions, and the stereoselective activity on antimyocardial ischemia, ocotillol-type saponins are subjected to extensive consideration. In this review, we sum up the discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins.]]> |
| [17] |
Bi Y, Ma C, Zhou Z W, Zhang T T, Zhang H Y, Zhang X C, Lu J, Meng Q G, Lewis P J, Xu J Y . Rec. Nat. Prod., 2015,9:356.
|
| [18] |
Bi Y, Yang J, Ma C, Liu Z Y, Zhang T T, Zhang X C, Lu J, Meng Q G . Pharmazie, 2015,70:213. https://www.ncbi.nlm.nih.gov/pubmed/26012249
PMID: 26012249 A series of novel ocotillol-type furoxan derivatives was synthesized by coupling various furoxans to 3-OH of 6-deoxy ocotillol, and their in vitro nitric oxide (NO) releasing capability was studied. The discharge of NO was examined after 30 min at two different concentrations, the results showed that all of the compounds tested could release NO in a dose-dependent manner. All of the synthesized compounds released similar amounts of NO at 100 μM, whereas at 500 μM these compounds showed more difference, in which compound II1, II3, II4, III2 displayed higher potency in releasing NO at this concentration. Analysis of the in vitro data showed that the derivatives bearing the same furoxan group on different ocotillol cores possessed various NO releasing capacity, suggesting that the structure of carrier of NO releasing groups may affect the NO release. Indeed, except compound II2, 24(S)-6-deoxy ocotillol derivatives from compound 6 with different furoxan substitutions at 3-OH and III2 displayed enhanced NO releasing capacity, compared to other compounds derived from compounds 5 and 9. The results illustrated that the functional group and the stereochemistry on the ocotillol structure may affect the NO release of furoxans. |
| [19] |
Kim S J, Shin J Y, Ko S K . J. Ginseng Res., 2016,40:86. https://www.ncbi.nlm.nih.gov/pubmed/26843826
DOI: 10.1016/j.jgr.2015.04.008 PMID: 26843826 This study compared the contents of prosapogenin depending on the extracting conditions of Red ginseng to provide basic information for developing Red ginseng-based functional foods. The content of ginsenoside Rg3 reached their maximum value at 24 h of extraction, followed by 36 h and 72 h of extraction at 100°C. |
| [20] |
Xia Y G, Liang J, Li G Y, Yang B Y, Kuang H X . J. Mass Spectrom., 2016,51:947. https://www.ncbi.nlm.nih.gov/pubmed/27383264
DOI: 10.1002/jms.3806 PMID: 27383264 - /ESI+ -FT-MS(1, 1) to MS(1, 4) spectra was constructed for the identification of structural elements in the MTSs. As a result, a total of 23 MTSs were discovered and tentatively identified, which had not been reported from Caulophyllum species before. All of these were potentially new compounds. This study provides an excellent example for discovery and identification of MTSs in herb medicines. Copyright © 2016 John Wiley & Sons, Ltd.]]> |
| [21] |
Hu Y P, Cui X M, Zhang Z J, Chen L J, Zhang Y M, Wang C X, Yang X Y, Qu Y, Xiong Y . Molecules, 2018,23:1206.
|
| [22] |
Munir M T, Kheirkhah H, Baroutian S, Quek S Y, Young B R . J. Clean. Prod., 2018,183:487.
|
| [23] |
Zhang Y, Li Y, Liu Z, Zhong L, Chi R, Yu J . Wuhan University Journal of Natural Sciences, 2015,20:247.
|
| [24] |
Zhong Z X, Li G K, Luo Z B, Zhu B H . Talanta, 2019,194:46. https://www.ncbi.nlm.nih.gov/pubmed/30609558
DOI: 10.1016/j.talanta.2018.09.105 PMID: 30609558 -1 and 0.85-11.0 mg kg-1, respectively. The recoveries ranged from 90.2% to 106.1% with relative standard deviations of 0.30-3.1%. The method was successfully applied to the analysis of cosmetics, in which all the colorants could be quantified, and their median values ranged from 4.94 to 591 mg kg-1 for seventy-two lipsticks, and from 7.70 to 1.73 × 103 mg kg-1 for fifty eye shadows, respectively. The proposed protocol could achieve batch preparation of samples and avoid measurement errors from the obvious volume reduction of the recovered extract, and it could serve as a powerful tool for high-throughput analysis of multiple colorants in complex samples.]]> |
| [25] |
Choi P, Park J Y, Kim T, Park S. H, Kim H. K, Kang K S, Ham J . J. Funct. Foods, 2015,14:613.
|
| [26] |
Wu W, Sun L, Zhang Z, Guo Y, Liu S . J. Pharmaceut. Biomed., 2015,107:141.
|
| [27] |
Lee J H, Ko M J, Chung M S . J. Supercrit. Fluids, 2018,133:177.
|
| [28] |
Wan H D, Li D . RSC Adv., 2015,5:78874.
|
| [29] |
Palaniyandi S A, Damodharan K, Lee K W, Yang S H, Suh J W . Biotechnol. Bioprocess Eng., 2015,20:608.
|
| [30] |
Ganzler K, Salgo A, Valko K . J. Chromatogr., 1986,371:299. https://www.ncbi.nlm.nih.gov/pubmed/3558551
DOI: 10.1016/s0021-9673(01)94714-4 PMID: 3558551 The applicability of microwave irradiation to the extraction of various types of compounds from soil, seeds, foods and feeds as a novel sample preparation method for chromatography was investigated. Samples were ground and mixed with an appropriate solvent, methanol or methanol-water for polar compounds and hexane for non-polar compounds. The suspensions were irradiated for 30 s, but they were not allowed to boil. After cooling, the irradiation was repeated several times. The samples were then centrifuged, and aliquots of the supernatant were injected into a chromatographic column. The yields of the extracted compounds obtained by microwave irradiation were compared with those obtained by the traditional Soxhlet or shake-flask extraction methods. The microwave extraction method was more effective than the conventional methods. Due to the considerable savings in time and energy, this novel method is suitable for fast extractions of large sample series. |
| [31] |
Yao H, Li X, Liu Y, Wu Q, Jin Y . J. Ginseng Res., 2016,40:415. https://www.ncbi.nlm.nih.gov/pubmed/27746695
DOI: 10.1016/j.jgr.2016.06.007 PMID: 27746695 Panax quinquefolius L. have strong bioactivities. The fact that it is hard to obtain large amounts of rare ginsenosides seriously restricts further research on these compounds. An easy, fast, and efficient method to obtain different kinds of rare ginsenosides simultaneously and to quantify each one precisely is urgently needed.]]> |
| [32] |
Yoon S H, Nam Y M, Hong J T, Kim S J, Ko S K . J. Ginseng Res., 2016,40:300. https://www.ncbi.nlm.nih.gov/pubmed/27616907
DOI: 10.1016/j.jgr.2015.09.001 PMID: 27616907 The result of USRG-12 indicated that ultrasonication-processed (100°C, 12 h) red ginseng extracts had the highest amount of ginsenosides Rg3 (0.803%), Rg5 (0.167%), and Rk1 (0.175%). |
| [33] |
Biswas T, Ajayakumar P V, Mathur A K, Mathur A Nat . Prod. Res., 2015,29:1256. https://www.ncbi.nlm.nih.gov/pubmed/25813381
DOI: 10.1080/14786419.2015.1024119 PMID: 25813381 The present study aims at developing an extraction protocol for efficient ginsenoside recovery from cell suspensions of Panax quinquefolius and P. sikkimensis. Methanol (100%, 70% and 30%), water (40°C, 90°C), water-saturated butanol and butanol-saturated water were compared for their ultrasonication-assisted ginsenoside retrieval efficacy. HPLC and HP-TLC analysis revealed 100% methanol as the best solvent for maximum retrieval of Rb (diol) and Rg (triol) ginsenosides (P. quinquefolius: Rb: 0.189, Rg: 3.163 mg/g DW; P. sikkimensis: Rb: 0.245, Rg: 4.073 mg/g DW), followed by water (90°C). Methanolic solutions, especially 70%, proved to be significant retrievers of Rg1 (1.812 and 1.327 mg/g DW in P. quinquefolius and P. sikkimensis), with poor Re recovery (0.328 and 0.342 mg/g DW). Water-saturated butanol also led to significant ginsenoside extraction (72.4% of content extracted by methanol), selectively in P. quinquefolius, with a less than 50% of total content extracted by methanol, in P. sikkimensis. |
| [34] |
Liu Z, Xia J, Wang C Z, Zhang J Q, Ruan C C, Sun G Z, Yuan C S . J. Agr. Food Chem., 2016,64:5389. https://www.ncbi.nlm.nih.gov/pubmed/27295137
DOI: 10.1021/acs.jafc.6b00963 PMID: 27295137 Panax ginseng contains many chemical components, including acidic ginsenosides and organic acids. However, whether these acidic substances play a role in ginsenoside transformation during steaming treatment has not yet been explored. In this paper, the content of neutral ginsenosides, acidic ginsenosides, and their degradation products in unsteamed and steamed P. ginseng were simultaneously quantified by high-performance liquid chromatography. We observed that neutral ginsenosides were converted to rare ginsenosides during the root steaming but not during the individual ginsenoside steaming. In contrast, acidic malonyl ginsenosides released malonic acid and acetic acid through demalonylation, decarboxylation, deacetylation reactions during the steaming at 120 °C. These malonyl ginsenosides not only were converted to rare ginsenosides but also promoted the degradation of neutral ginsenosides. Further studies indicated that a low concentration of organic acid was the determining factor for the ginsenoside conversion. The related mechanisms were deduced to be mainly acidic hydrolysis and dehydration. In summary, acidic ginsenosides and organic acids remarkably affected ginsenoside transformation during the steaming process. Our results provide useful information for precisely understanding the ginsenoside conversion pathways and mechanisms underlying the steaming process. |
| [35] |
Cui Q, Liu J, Xu W, Kang Y F, Wang X, Li Y, Fu Y J . Clean. Prod., 2019,210:1507. https://linkinghub.elsevier.com/retrieve/pii/S0959652618335455
|
| [36] |
Zhang Y C, Zhang J X, Liu C M, Yu M, Li S N . J. Chromatogr. A, 2017,1483:20. https://www.ncbi.nlm.nih.gov/pubmed/28027838
DOI: 10.1016/j.chroma.2016.12.068 PMID: 28027838 1, Rg5, Rs5, 20R-Rg3, and Rs4 exceeded 50.00%; indicating that the aforementioned chemical compounds have potential for further development. The results were validated by comparison with authentic standards, indicating that the method used in this research was accurate and advantageous for matrix analysis.]]> |
| [37] |
Wang Y H, Li Y, Zhang Y, Feng G, Yang Z X, Guan Q X, Wang R, Han F J . Molecules, 2017,22:17.
|
| [38] |
Sunwoo H H, Gujral N, Huebl A C, Kim C T . Food Bioprocess Tech., 2014,7:1246. http://link.springer.com/10.1007/s11947-013-1234-1
|
| [39] |
Zhong F L, Ma R, Jiang M L, Dong W W, Jiang J, Wu S Q, Li D H, Quan L H . J. Microbiol. Biotechnol., 2016,26:1661. https://www.ncbi.nlm.nih.gov/pubmed/27435543
DOI: 10.4014/jmb.1605.05052 PMID: 27435543 bgy2) was cloned from Lactobacillus brevis. We expressed this gene in Escherichia coli BL21(DE3), isolated the resulting protein, and then utilized the enzyme for the biotransformation of ginsenosides. The bgy2 gene contains 2,223 bp, and encodes a protein of 741 amino acids that is a member of glycosyl hydrolase family 3. β-Glucosidase (Bgy2) cleaved the outer glucose moieties of ginsenosides at the C-20 position, and the inner glucose at the C-3 position. Under optimal conditions (pH 7.0, 30°C), we used 0.1 mg/ml Bgy2 in 20 mM sodium phosphate buffer (PBS) for enzymatic studies. In these conditions, 1.0 mg/ml ginsenoside Rb1 and ginsenoside F2 were converted into 0.59 mg/ml ginsenoside Rd and 0.72mg/ml compound K, with molar conversion productivities of 69% and 91%, respectively. In pharmaceutical and commercial industries, this recombinant Bgy2 would be suitable for producting ginsenoside Rd and compound K.]]> |
| [40] |
Liu F, Ma N, He C W, Hu Y J, Li P, Chen M W, Su H X, Wan J B . J. Ginseng Res., 2018,42:149. https://www.ncbi.nlm.nih.gov/pubmed/29719461
DOI: 10.1016/j.jgr.2017.01.007 PMID: 29719461 Panax notoginseng leaves (PNL) exhibit extensive activities, but few analytical methods have been established to exclusively determine the dammarane triterpene saponins in PNL.]]> |
| [41] |
Wan J Y, Wang C Z, Liu Z, Zhang Q H, Musch M W, Bissonnette M, Chang E B, Li P, Qi L W, Yuan C S . J. Chromatogr. B, 2016,1015:62.
|
| [42] |
Zheng H R, Chu Y, Zhou D Z, Ju A C, Li W, Li X, Xia Y, Polachi N, Li D K, Zhou S P, Sun H, Liu C X . J. Chromatogr. B, 2018,1072:282. https://www.ncbi.nlm.nih.gov/pubmed/29202359
DOI: 10.1016/j.jchromb.2017.10.056 PMID: 29202359 1, Rd, Re and Rh1, 2.5ng/mL for ginsenoside Rf, Rg3, Rb2 and Rb3 and 5.0ng/mL for ginsenoside Rb1 and Rc, respectively. All the precision (RSD) data ranged from 1.7-14.5% and the accuracy (RE) data was within ±13.73%. Moreover, the validated method has been applied to investigate the integrated pharmacokinetic profiles of ginsenosides in CHF rats following intravenous administration of YQFM successfully.]]> |
| [43] |
Yang Q, Li J H, Wang X Y, Xiong H, Chen L X . Anal. Chem., 2019,91:6561. https://www.ncbi.nlm.nih.gov/pubmed/31010290
DOI: 10.1021/acs.analchem.9b00082 PMID: 31010290 A novel ternary-emission fluorescence sensor was proposed by post-imprinting mixing blue-/green-/red-emission bovine hemoglobin (BHb) imprinted polymers (b-MIPs, g-MIPs, and r-MIPs) at a proper ratio and realized the multiplexed and visual detection of BHb. The three MIPs were individually embedded with blue-emission 7-hydroxycoumarin, green-emission CdTe quantum dots (QDs), and red-emission CdTe/ZnS QDs. Upon interaction with BHb within 8 min, the fluorescence of CdTe and CdTe/ZnS QDs were simultaneously turned off, whereas the 7-hydroxycoumarin turned on the fluorescence intensity. Thereupon, the ratiometric fluorescence intensity of the ternary emission linearly varied within 0.025-3 μM BHb, accompanying the profuse fluorescence color evolution from yellowish green to yellow to salmon to plum to purple to finally blue. In comparison with the dual- or single-emission sensor, the ternary-emission fluorescence MIPs sensor provided a wider color variation covering the green-red-blue window for accurate naked-eye determination of BHb, as well as a lower detection limit down to 7.8 nM and a higher imprinting factor of 15.2. Moreover, the satisfactory recoveries of 99.25-111.7% in determining the spiked BHb in bovine urine samples, as well as the optical stability and post-imprinting construction convenience, indicated that the developed tricolor-emission fluorescence MIPs sensor provided an ideal alternative for rapid, sensitive, and visual determination of proteins in complicated samples. |
| [44] |
明魏娜(Ming W N), 王晓艳(Wang X Y), 明永飞(Ming Y F), 李金花(Li J H), 陈令新(Chen L X) . 化学进展 (Progress in Chemistry), 2016,28:552.
|
| [45] |
Wang L Y, Li J H, Wang J N, Guo X T, Wang X Y, Choo J, Chen L X . J. Colloid Interf. Sci., 2019,541:376. https://www.ncbi.nlm.nih.gov/pubmed/30710820
DOI: 10.1016/j.jcis.2019.01.081 PMID: 30710820 Green ion imprinted polymers (IIPs) were prepared in aqueous phase via the synergy of three functional monomers of low-cost eco-friendly gelatin (G), 8-hydroxyquinoline (HQ) and chitosan (C), namely G-HQ-C IIPs, and were applied as an effective and recyclable adsorbent to remove Cu(II) from aqueous solution. The as-prepared G-HQ-C IIPs were systematically characterized, and several major factors affecting adsorption capacity including solution pH, temperature and contact time were investigated in detail. The adsorption of Cu(II) on G-HQ-C IIPs followed the pseudo-second-order kinetic and Langmuir isotherm models, and the adsorption capacity increased with temperature increase. Moreover, the maximum adsorption capacities of G-HQ-C IIPs toward Cu(II) reached up to 111.81 mg/g at room temperature, much higher than those of most of the reported adsorbents for Cu(II). The G-HQ-C IIPs displayed excellent selectivity against seven common divalent ions with selectivity coefficients above 18.71, as well as high anti-interference ability. Additionally, a good reusability was demonstrated without significant loss in adsorption capacity after at least ten cycles. The IIPs were applied to environmental water samples for selective removal of Cu(II) with satisfactory results. By replacing Cu(II) template by Cd(II), Hg(II) and Pb(II), respectively, the obtained three kinds of IIPs based on G-HQ-C presented convincing imprinting properties, and therefore the work could provide a simple and general imprinting strategy toward various concerned heavy metal ions through multi-point interactions from multiple functional monomers. |
| [46] |
Xing R R, Wen Y R, Dong Y R, Wang Y J, Zhang Q, Liu Z . Anal. Chem., 2019,91:9993. https://www.ncbi.nlm.nih.gov/pubmed/31347834
DOI: 10.1021/acs.analchem.9b01826 PMID: 31347834 2 nanoparticles were prepared as nanotags for the specific labeling of captured protein. The formed MIP-protein-MIP sandwich-like complexes could produce a significantly enhanced SERS signal. The dual MIP-based recognitions ensured high specificity of the assay, while SERS detection provided ultrahigh sensitivity. The duMIP-PISA of neuron-specific enolase (NSE) in human serums was demonstrated, which permitted the differentiation of small cell lung cancer patients from healthy individuals. As compared to regular ELISA, the duMIP-PISA exhibited multiple merits including a simpler procedure, faster speed, lower sample volume requirement, and wider linear range. The approach well demonstrated the great potentials of MIPs and can be easily modified and extended to other protein biomarkers. Therefore, the duMIP-PISA approach holds great promise in many important applications such as disease diagnosis.]]> |
| [47] |
BelBruno J J . Chem. Rev., 2019,119:94. https://www.ncbi.nlm.nih.gov/pubmed/30246529
DOI: 10.1021/acs.chemrev.8b00171 PMID: 30246529 Molecularly imprinted polymers are synthetic receptors for a targeted molecule. As such, they are analogues of the natural antibody-antigen systems. In this review, after a recounting of the early history of the general field, we specifically focus on the application of these polymers as sensors. In these applications, the polymers are paired with a reporting system, which may be electrical, electrochemical, optical, or gravimetric. The presence of the targeted molecule effects a change in the reporting agent, and a calibrated quantity of the target is recorded. In this review, we describe the imprinted polymer production processes, the techniques used for reporting, and the applications of the reported sensors. A brief survey of recent applications to gas-phase sensing is included, but the focus is primarily on the development of sensors for targets in solution. Included among the applications are those designed to detect toxic chemicals, toxins in foods, drugs, explosives, and pathogens. The application of computational chemistry to the development of new imprinted polymers is included as is a brief assessment of future developments. |
| [48] |
Culver H R, Peppas N A . Chem. Mat., 2017,29:5753.
|
| [49] |
张伟(Zhang W), 孙成贺(Sun C H), 王绍艳(Wang S Y), 李芊(Li Q)1, 王英平(Wang Y) . 精细化工 (Fine Chemicals), 2015,32:1102.
|
| [50] |
Sun C H, Wang J H, Huang J J, Yao D D, Wang C Z, Zhang L, Hou S Y, Chen L N, Yuan C S . Polymers, 2017,9:18.
|
| [51] |
Liu Q S, He J, Zhou W B, Gu Y L, Huang H Q, Li K Q, Yin X Y . J. Sep. Sci., 2017,40:744. https://www.ncbi.nlm.nih.gov/pubmed/27935252
DOI: 10.1002/jssc.201601193 PMID: 27935252 18 solid-phase extraction column. Overall, a new, innovative method was developed to efficiently enrich high-polarity bioactive molecules present at low concentrations in complex matrices.]]> |
| [52] |
Cai Q Z, Yang Z Y, Chen N, Zhou X M, Hong J L . J. Chromatogr. A, 2016,1455:65. https://www.ncbi.nlm.nih.gov/pubmed/27295967
DOI: 10.1016/j.chroma.2016.05.089 PMID: 27295967 In the present work, an advanced pretreatment method magnetic molecular imprinted polymers-dispersive solid phase extraction (MMIPs-DSPE) combined with the high sensitivity LTQ-Orbitrap mass spectrometry was applied to the complicated metabolites analysis of Traditional Chinese Medicines (TCMs) in complex matrices. The ginsenoside Rb1 magnetic molecular imprinted polymers (Rb1-MMIPs) were successfully synthesized for specific recognition and selective enrichment of Panax notoginseng saponin metabolites in rat faeces. The polymers were prepared by using Fe3O4@SiO2 as the supporting material, APTES as the functional monomer and TEOS as the cross-linker. The Rb1-MMIPs showed quick separation (10.8 emu/g), large adsorption capacity (636μmol/g), high selectivity and fast binding kinetics (25min). Dispersion solid-phase extraction using Rb1-MMIPs (Rb1-MMIPs-DSPE) integrated with LTQ-Orbitrap MS was applied to fish out and identify saponin metabolites from rat faeces, and totally 58 related compounds were detected within 20min, including 26 PPD-group and 32 PPT-group notoginsenoside metabolites. Parallel tests showed that Rb1-MMIPs-DSPE obtained the lowest matrix effects of 0.98-14.84% and captured the largest number of structural analogues compared with traditional pretreatment methods organic solvent extraction (OSE) and solid phase extraction (SPE). |
| [53] |
李健(Li J), 官亦标(Guan Y B), 傅凯(Fu K), 苏岳锋(Su Y F), 包丽颖(Bao L Y), 吴锋(Wu F) . 化学进展( Progress in Chemistry), 2014,26:1233. 10b2a9eb-4495-4417-9d72-ab630532eba9 http://www.progchem.ac.cn//CN/abstract/abstract11392.shtml
DOI: 10.7536/PC140227 当今社会日益增长的能源与环境需求对储能电池技术的发展既是机遇也是严峻的挑战。纳米碳材料如碳纳米管与石墨烯因其优异的导电能力、良好的机械性能以及独特的形貌与结构特征在储能电池技术领域中的应用越来越普遍。本文通过综述近年来碳纳米管与石墨烯分别作为锂离子电池的复合电极材料、负极活性材料、导电添加剂以及新型锂硫电池用复合导电载体的最新应用进展,重点讨论了这两类纳米碳材料的不同应用模式对储能电池容量性能、倍率性能以及循环寿命的影响。同时对目前研究中存在的问题进行了总结,并对未来发展方向,如开发低成本与环境友好的高质量材料合成技术、提升材料的分散能力以有效构筑复合电极结构以及开发新的应用模式等进行了展望。 |
| [54] |
韩强(Han Q), 王宗花(Wang Z), 张晓琼(Zhang X), 丁明玉(Ding M) . 化学进展( Progress in Chemistry), 2014,26:820. 758efe2b-7014-4c6e-a098-8e292f8a8cf0 http://www.progchem.ac.cn//CN/abstract/abstract11356.shtml
DOI: 10.7536/PC131145 样品前处理新技术与方法研究是现代分析化学的重要研究课题与发展方向之一。固相萃取是目前应用最为广泛的样品前处理技术,其技术核心是吸附材料,因此开发新型吸附材料是样品前处理领域的研究热点。石墨烯是一种新型碳纳米材料,由于其良好的物理化学性质,在短短几年内迅速成为众多学科的研究热点。其高比表面积、良好的化学稳定性和热稳定性使之在分离科学领域得到广泛的应用。本文系统综述了石墨烯及其复合材料在样品前处理中的应用研究,主要包括其作为固定相在固相萃取、固相微萃取、磁固相萃取等技术在环境、食品、生物等样品前处理中的应用。 |
| [55] |
苗瑞(Miao R), 吴冬雪(Wu D X), 王秋颖(Wang Q Y), 赵幻希(Zhao H X), 李雪(Li X), 修洋(Xiu Y), 刘淑莹(Liu S Y) . 高等学校化学学报 (Chemical Journal of Chinese Universities), 2018,39:2178.
|
| [56] |
He Y D, Wei Y Q, Sun X J, Zhou G W, Zheng J . Anal. Methods, 2018,10:2464.
|
| [57] |
Peng J J, Li D X, Huang J Y, Tong L, Yu B Y . Chin. Herb. Med., 2017,9:267.
|
| [58] |
Wang H P, Zhang Y B, Yang X W, Zhao D Q, Wang Y P . J. Ginseng Res., 2016,40:382. https://www.ncbi.nlm.nih.gov/pubmed/27746691
DOI: 10.1016/j.jgr.2015.12.001 PMID: 27746691 Panax ginseng (GRR). This study was carried out to qualitatively and quantitatively determine the ginsenosides in the cultivated and forest GRR.]]> |
| [59] |
Du Z X, Li J H, Zhang X, Pei J, Huang L F . Molecules, 2018,23:20.
|
| [60] |
Liang Z T, Chen Y J, Xu L, Qin M J, Yi T, Chen H B, Zhao Z Z . J. Pharmaceut. Biomed., 2015,105:121. https://linkinghub.elsevier.com/retrieve/pii/S0731708514006001
|
| [61] |
He C Y, Feng R, Sun Y P, Chu S F, Chen J, Ma C, Fu J, Zhao Z X, Huang M, Shou J W, Li X Y, Wang Y Z, Hu J F, Wang Y, Zhang J T . Acta Pharm. Sin. B, 2016,6:593. https://www.ncbi.nlm.nih.gov/pubmed/27818927
DOI: 10.1016/j.apsb.2016.05.001 PMID: 27818927 r>0.995) within the determined ranges. Both intra-day and inter-day variances were less than 15%, and the accuracy was within 80-120%. The excretion recoveries of Rg1, ginsenoside Rh1 (Rh1), and protopanaxatriol (Ppt) in bile, urine, and feces combined were all greater than 70%. The fecal excretion recoveries of Rg1, Rh1, and Ppt were 40.11%, 22.19%, and 22.88%, respectively, whereas 6.88% of Rg1 and 0.09% of Rh1 were excreted in bile. Urinary excretion accounted for only 0.04% of Rg1. In conclusion, the observed excretion profiles for Rg1 and its metabolites after oral administration are helpful for understanding the poor oral bioavailability of Rg1 and will aid further investigations of Rg1 as a pharmacologically active component.]]> |
| [62] |
Li P, Zhao P Y, Liu W J, Jiang Y F, Wang W J, Bao L Y, Jin Y R, Li X W . Microchem. J., 2018,137:302.
|
| [63] |
Wang Y L, Sha C J, Liu W H, Gai Y Y, Zhang H Y, Qu H L, Wang W S . J. Pharmaceut. Biomed., 2012,62:87.
|
| [64] |
Biswas T, Kalra A, Mathur A K, Lal R K, Singh M, Mathur A . Appl. Microbiol. Biot., 2016,100:4909. https://www.ncbi.nlm.nih.gov/pubmed/26795963
DOI: 10.1007/s00253-015-7264-z PMID: 26795963 Cobalt nitrate, nickel sulphate, hydrogen peroxide, sodium nitroprusside, and culture filtrates of Pseudomonas monteili, Bacillus circularans, Trichoderma atroviridae, and Trichoderma harzianum were tested to elicit ginsenoside production in a cell suspension line of Panax quinquefolius. Abiotic elicitors preferentially increased panaxadiols whereas biotic elicitors upregulated the panaxatriol synthesis. Cobalt nitrate (50 μM) increased total ginsenosides content by twofold (54.3 mg/L) within 5 days. It also induced the Rc synthesis that was absent in the control cultures. Elicitation with P. monteili (2.5 % v/v, 5 days) also supported 2.4-fold enhancement in saponin yield. Elicitation by T. atroviridae or hydrogen peroxide induced the synthesis of Rg3 and Rh2 that are absent in ginseng roots. The highest ginsenosides productivity (3.2-fold of control) was noticed in cells exposed to 1.25 % v/v dose of T. atroviridae for 5 days. Treating cells with T. harzianum for 15 days afforded maximum synthesis and leaching (8.1 mg/L) of ginsenoside Rh1. |
| [65] |
Zhang J J, Su H, Zhang L, Liao B S, Xiao S M, Dong L L, Hu Z G, Wang P, Li X W, Huang Z H, Gao Z M, Zhang L J, Shen L, Cheng R Y, Xu J, Chen S L . Molecules, 2017,22:13. http://www.mdpi.com/1420-3049/22/1/13
|
| [66] |
Yunusova N, Kim J Y, Lee G J, Hong J Y, Shin B K, Cai S Q, Piao X L, Park J H, Kwon S W . Int. J. Food Sci. Tech., 2015,50:1607.
|
| [67] |
Lee G J, Shin B K, Yu Y H, Ahn J, Kwon S W, Park J H . J. Pharmaceut. Biomed., 2016,128:158. https://linkinghub.elsevier.com/retrieve/pii/S0731708516302692
|
| [68] |
Yu H S, Wang Y, Liu C Y, Yang J M, Xu L Q, Li G H, Song J G, Jin F X . Chem. Pharm. Bull., 2018,66:901. https://www.ncbi.nlm.nih.gov/pubmed/30175750
DOI: 10.1248/cpb.c18-00426 PMID: 30175750 3 catalysis, the reaction conditions are similar to enzymatic reaction conditions. In FeCl3 catalysis, the only 20-O-sugar-moiety of ginsenoside Rb1 was decomposed into the minor ginsenosides Rk1 and Rg5 with newly produced C-20 ethylene bands; but also hydrolyzed into 20(S)-Rg3 and 20(R)-Rg3; subsequently the C-24(25) ethylene bands of 20(S)-Rg3 and 20(R)-Rg3 were hydrated to 20(S)-25-OH-Rg3 and 20(R)-25-OH-Rg3. After separation of reaction mixture from 34 g ginsenoside-Rb1 by silica-gel-column, the 3.3 g sample I of TLC top-band consisting of Rg5 and Rk1, 8.7 g sample II of TLC middle-band consisting of 20(S)-Rg3 and 20(R)-Rg3, 3.5 g sample III of TLC bottom-band consisting of unknown product-I and -II including 20(S)-25-OH-Rg3, were obtained. The sample III consisting of unknown product-I and -II was purified by crystallization, and identified to 20(S)-25-OH-Rg3 and 20(R)-25-OH-Rg3 by HPLC-Evaporative Light Scattering Detector (ELSD) and NMR. Therefore, six types of minor-ginsenosides Rk1, Rg5, 20(S)-Rg3, 20(R)-Rg3, 20(S)-25-OH-Rg3 and 20(R)-25-OH-Rg3 were successfully prepared from ginsenoside Rb1 by FeCl3 catalysis. FeCl3 has low toxicity and is inexpensive, and the reaction conditions are similar to enzymatic reaction conditions; thus, this method is applicable to the development of ginseng-based drugs.]]> |
| [69] |
Guo C, Li D M, Liu C M, Guo Z P, Chen Y . Anal. Bioanal. Chem., 2018,410:4293. https://www.ncbi.nlm.nih.gov/pubmed/29748756
DOI: 10.1007/s00216-018-1078-7 PMID: 29748756 2, cholic acid, and chenodeoxycholic acid in saliva. The method consists of two successive steps: fast and direct labeling of the target analytes with N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide followed by ultrahigh-performance liquid chromatography-tandem mass spectrometry analysis. The method exhibited a wide linear range from 2.5 to 2500 pg/mL, with linear coefficients greater than 0.9963 and limits of detection and quantification as low as 0.10 and 0.33 pg/mL, respectively. The method precision was evaluated, with relative standard deviations ranging from 2.12% to 10.63% for intraday assays and from 2.98% to 12.88% for interday assays. The recoveries were measured by our spiking saliva samples with standards at three different levels, and ranged from 72.5% to 98.0%. Real applicability was validated by direct quantification of trace target analytes in human saliva, with simple pretreatment, use of a small sample volume, and a short analysis time. Graphical abstract Sequential steps to extract, label, and determine the ultratrace carboxylic acids in saliva. CDCA chenodeoxycholic acid, γ-CEHC 2-(β-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman, α-LA α-lipoic acid, PGE2 prostaglandin E2, UHPLC-MS/MS ultrahigh-performance liquid chromatography-tandem mass spectrometry.]]> |
| [70] |
张俊杰(Zhang J J), 贾金萍(Jia J P), 秦雪梅(Qin X M) . 分析测试学报 (Journal of Instrumental Analysis), 2017,36:579.
|
| [71] |
Cheng C S, Wu W R, Huang B M, Liu L, Luo P, Zhou H . Phytochem. Lett., 2016,17:194.
|
| [72] |
Wu W, Jiao C X, Li H, Ma Y, Jiao L L, Liu S Y . Phytochem. Anal., 2018,29:331. https://www.ncbi.nlm.nih.gov/pubmed/29460310
DOI: 10.1002/pca.2752 PMID: 29460310 Panax ginseng has received much attention as a valuable health supplement with medicinal potential. Its chemical diversity and multiple pharmacological properties call for comprehensive methods to better understand the effects of ginseng and ginsenosides. Liquid chromatography-mass spectrometry (LC-MS) based metabonomic approaches just fit the purpose. |
| [73] |
Wang Y P, Choi H K, Brinckmann J A, Jiang X, Huang L F . J. Chromatogr. A, 2015,1426:1. https://www.ncbi.nlm.nih.gov/pubmed/26643719
DOI: 10.1016/j.chroma.2015.11.012 PMID: 26643719 Panax quinquefolius (PQ) is one of the best-selling natural health products due to its proposed beneficial anti-aging, anti-cancer, anti-stress, anti-fatigue, and anxiolytic effects. In recent years, the quality of PQ has received considerable attention. Sensitive and accurate methods for qualitative and quantitative analyses of chemical constituents are necessary for the comprehensive quality control to ensure the safety and efficacy of PQ. This article reviews recent progress in the chemical analysis of PQ and its preparations. Numerous analytical techniques, including spectroscopy, thin-layer chromatography (TLC), gas chromatography (GC), high-performance liquid chromatography (HPLC), liquid chromatography/mass spectrometry (LC/MS), high-speed centrifugal partition chromatography (HSCPC), high-performance counter-current chromatography (HPCCC), nuclear magnetic resonance spectroscopy (NMR), and immunoassay, are described. Among these techniques, HPLC coupled with mass spectrometry (MS) is the most promising method for quality control. The challenges encountered in the chemical analysis of PQ are also briefly discussed, and the remaining questions regarding the quality control of PQ that require further investigation are highlighted. |
| [74] |
Liu J, Liu Y, Wang Y, Abozeid A, Zu Y G, Tang Z H . J. Pharmaceut. Biomed., 2017,135:176. https://linkinghub.elsevier.com/retrieve/pii/S0731708516314613
|
| [75] |
Cui S Q, Wang J, Yang L C, Wu J F, Wang X L . J. Pharmaceut. Biomed., 2015,102:64.
|
| [76] |
Yang L, Yu Q T, Ge Y Z, Zhang W S, Fan Y, Ma C W, Liu Q, Qi L W . Sci. Rep., 2016,6:11. https://www.ncbi.nlm.nih.gov/pubmed/28442704
DOI: 10.1038/s41598-016-0013-4 PMID: 28442704 i = 883 nM was discovered. This compound exhibited low cell toxicity and was able to selectively inhibit shedding of known ADAM10 substrates in several cell-based models. We hypothesize that differential glycosylation of these cognate substrates is the source of selectivity of our novel inhibitor. The data indicate that this novel inhibitor can be used as an in vitro and, potentially, in vivo, probe of ADAM10 activity. Additionally, results of the present and prior studies strongly suggest that glycosylated substrate are applicable as screening agents for discovery of selective ADAM probes and therapeutics.]]> |
| [77] |
Liu J, Liu Y, Wang Y, Abozeid A, Zu Y G, Zhang X N, Tang Z H . Molecules, 2017,22:14.
|
| [78] |
Park S E, Seo S H, Lee K I, Na C S and Son H S J . Ginseng Res., 2018,42:57. https://www.ncbi.nlm.nih.gov/pubmed/29348723
DOI: 10.1016/j.jgr.2016.12.010 PMID: 29348723 Ginseng contains many small metabolites such as amino acids, fatty acids, carbohydrates, and ginsenosides. However, little is known about the relationships between microorganisms and metabolites during the entire ginseng fermentation process. We investigated metabolic changes during ginseng fermentation according to the inoculation of food-compatible microorganisms. |
| [79] |
Sun Y F, Chen S Q, Wei R M, Xie X, Wang C C, Fan S H, Zhang X, Su J, Liu J, Jia W, Wang X Y . Food Funct., 2018,9:3547. https://www.ncbi.nlm.nih.gov/pubmed/29896600
DOI: 10.1039/c8fo00025e PMID: 29896600 Ginseng, a widely used functional food and food additive, has been proven to have promotion effects of health on the body. However, whether the long-term intake of Ginseng is beneficial or has side effects on an organism is still unclear. In this study, untargeted GC-TOFMS metabolomic analysis of serum, cecum and ileum intestinal contents was conducted to understand the effect of the long-term intake of Ginseng extracts. 16S rRNA microbial sequencing technology was applied to investigate the effect of Ginseng extracts on the structure of gut microbiota. Cytokines in spleen were detected to determine the effect of Ginseng extracts on the immune system. Compared to control groups, the metabolites in serum, cecum and ileum, such as amino acids, amines and other metabolites related to carbohydrate metabolism, significantly varied between the C and GS groups. Ginseng extracts affected the structure of gut microbiota with a decreased abundance of TM7, while the abundance of Proteobacteria, Methylobacteriaceae, Parasutterella, Sutterella increased in the GS group. The increased abundance of Bifidobacterium and Lactobacillus demonstrated that Ginseng extracts contribute to probiotic amplification. Highly correlated with Bifidobacterium and Lactobacillus, interleukin 4 (IL4), IL10 and immunoglobulin A (IgA) levels were significantly elevated after the long-term intake of Ginseng extracts. These results indicated that the long-term administration of Ginseng extracts positively affected the host-gut metabolism, immune system, the anti-inflammation process and the gut intestinal microbiota structure. |
| [80] |
Chang K H, Jo M N, Kim K T, Paik H D . J. Ginseng Res., 2014,38:47. c83995f3-6a1e-4331-83a2-5319de937469 https://www.ncbi.nlm.nih.gov/pubmed/24558310
DOI: 10.1016/j.jgr.2013.11.008 PMID: 24558310 The transformation of ginsenoside Rb1 into a specific minor ginsenoside using Aspergillus niger KCCM 11239, as well as the identification of the transformed products and the pathway via thin layer chromatography and high performance liquid chromatography were evaluated to develop a new biologically active material. The conversion of ginsenoside Rb1 generated Rd, Rg3, Rh2, and compound K although the reaction rates were low due to the low concentration. In enzymatic conversion, all of the ginsenoside Rb1 was converted to ginsenoside Rd and ginsenoside Rg3 after 24 h of incubation. The crude enzyme (β-glucosidase) from A. niger KCCM 11239 hydrolyzed the β-(1→6)-glucosidic linkage at the C-20 of ginsenoside Rb1 to generate ginsenoside Rd and ginsenoside Rg3. Our experimental demonstration showing that A. niger KCCM 11239 produces the ginsenoside-hydrolyzing β-glucosidase reflects the feasibility of developing a specific bioconversion process to obtain active minor ginsenosides. |
| [81] |
Yan X, Zhao Y, Zhang Y, Qu H H . Molecules, 2017,22:29.
|
| [82] |
Bai H R, Wang S J, Liu J J, Gao D, Jiang Y Y, Liu H X, Cai Z W . J. Chromatogr. B, 2016,1026:263. https://www.ncbi.nlm.nih.gov/pubmed/26520809
DOI: 10.1016/j.jchromb.2015.09.024 PMID: 26520809 The root of Panax ginseng C.A. Mey. (P. ginseng) is one of the most popular traditional Chinese medicines, with ginsenosides as its main bioactive components. Because different ginsenosides have varied pharmacological effects, extraction and separation of ginsenosides are usually required for the investigation of pharmacological effects of different ginsenosides. However, the contents of ginsenosides vary with the ages and tissues of P. ginseng root. In this research, an efficient method to explore the distribution of ginsenosides and differentiate P. ginseng roots with different ages was developed based on matrix assisted laser desorption/ionization time-of-flight mass spectrometry imaging (MALDI-TOF-MSI). After a simple sample preparation, there were 18 peaks corresponding to 31 ginsenosides with distinct localization in the mass range of m/z 700-1400 identified by MALDI-TOF-MSI and MALDI-TOF-MS/MS. All the three types of ginsenosides were successfully detected and visualized in images, which could be correlated with anatomical features. The P. ginseng at the ages of 2, 4 and 6 could be differentiated finely through the principal component analysis of data collected from the cork based on the ion images but not data from the whole tissue. The experimental result implies that the established method for the direct analysis of metabolites in plant tissues has high potential for the rapid identification of metabolites and analysis of their localizations in medicinal herbs. Furthermore, this technique also provides valuable information for the component-specific extraction and pharmacological research of herbs. |
| [83] |
Buchberger A R, DeLaney K, Johnson J, Li L J . Anal. Chem., 2018,90:240. https://www.ncbi.nlm.nih.gov/pubmed/29155564
DOI: 10.1021/acs.analchem.7b04733 PMID: 29155564 |
/
| 〈 |
|
〉 |