

文章编号: 20200112
文献标识码: A
界面钝化策略:提高钙钛矿太阳能电池的稳定性
收稿日期:2019-06-04
要求修回日期:2019-09-03
网络出版日期:2019-10-22
基金资助
国家重点研发计划(2016YFE0114900)
国家自然科学基金项目资助(21761132007)
版权
Interface Passivation Strategy: Improving the Stability of Perovskite Solar Cells
Received:4 Jun. 2019
rev-requestrev-request:3 Sept. 2019
Online:22 Oct. 2019
Fund
National Key R&D Program of China(2016YFE0114900)
National Natural Science Foundation of China(21761132007)
Copyright
近年来,新兴起的有机无机杂化钙钛矿太阳能电池突飞猛进,在短短十年里其光电转化效率从3.8%迅速发展到目前25.2%的认证效率,被视为最具有应用潜力的新型高效率太阳能电池之一。虽然钙钛矿太阳能电池具有很高的光电转换效率已与多晶硅薄膜电池相媲美,但是电池的长期稳定性仍是阻碍其商业化的一大挑战。钙钛矿表面和晶界存在大量的缺陷,界面钝化来提高钙钛矿太阳能电池的稳定性是非常重要且有效的策略。二维钙钛矿材料是有机胺层与无机层交替的层状钙钛矿,具有体积较大的有机铵阳离子,与传统的三维钙钛矿材料相比对于环境的稳定性较好,并且结构灵活可调,在三维钙钛矿表面修饰二维钙钛矿层钝化缺陷,在提高钙钛矿太阳能电池效率的同时又保证了稳定性,另外,合适的钝化剂分子也能够非常有效地钝化缺陷。本文总结了钙钛矿太阳能电池的不稳定因素,归纳了钙钛矿太阳能电池界面钝化方面的研究进展,指出了二维钙钛矿材料发展的巨大潜力以及寻找合适钝化剂分子的原则,期望能够为获得高性能的钙钛矿太阳能电池进而实现商业化提供有益的指导。

王蕾 , 周勤 , 黄禹琼 , 张宝 , 冯亚青 . 界面钝化策略:提高钙钛矿太阳能电池的稳定性[J]. 化学进展, 2020 , 32(1) : 119 -132 . DOI: 10.7536/PC190603
Lei Wang , Qin Zhou , Yuqiong Huang , Bao Zhang , Yaqing Feng . Interface Passivation Strategy: Improving the Stability of Perovskite Solar Cells[J]. Progress in Chemistry, 2020 , 32(1) : 119 -132 . DOI: 10.7536/PC190603
In recent years, the emerging organic and inorganic hybrid perovskite solar cells have made rapid progress. In just ten years, its photoelectric conversion efficiency has rapidly developed from 3.8% to the current certified efficiency of 25.2%, which is regarded as one of the most potential solar cells. Although perovskite solar cells have high photoelectric conversion efficiency comparable to polysilicon thin film cells, the long-term stability of the cells remains a major challenge hindering their commercialization. There are many defects on the surface and grain boundary of perovskite. Interface passivation is an important and effective strategy to improve the stability of perovskite solar cells. Two-dimensional perovskite materials are organic amine and inorganic layer alternate layered perovskite, with bulky organic ammonium cations. Compared with the traditional three-dimensional perovskite materials, the stability for the environment is good, with the flexible and adjustable structure. The 3D perovskite’s surface is modified by a two-dimensional perovskite to passivate defects, ensuring the stability and at the same time improving the efficiency of perovskite solar cells. In addition, suitable passivation agent molecules can also passivate defects effectively. This paper reviews the unstable factors of perovskite solar cells, summarizes the research progress in interface passivation of perovskite solar cell, points out the great potential of two-dimensional perovskite materials’ development and the principle of finding suitable passivation agent molecules, which is expected to provide useful guidance for obtaining high-performance perovskite solar cells and realizing commercialization.
Key words: perovskite solar cell ; stability ; interface passivation
图1 (a) 三维混合钙钛矿ABX3结构图,显示角共享[BX6]4-八面体(A是有机阳离子,B是金属阳离子,X是卤化物);(b) 在潮湿、紫外光或热作用下,3D钙钛矿可分解为前体材料或0D水化相;(c) 显示了在三维钙钛矿中,O2通过晶粒渗透而开始的降解过程[23]Fig. 1 (a) Illustration of the 3D hybrid perovskite structure ABX3, showing the corner-sharing [BX6]4- octahedra (A is an organic cation, B is a metal cation and X is a halide); (b) Upon exposure to moisture, UV light or heat, 3D perovskites decompose into either the precursor materials or a 0D hydrated phase; (c) Illustration showing the degradation processes initiated by infiltration of O2 through the grains in a 3D perovskite[23] (Reproduced with permission from ref 23) |
图4 (a) BA处理钙钛矿薄膜形成2D/3D堆积结构,(b,c) BA和BAI处理钙钛矿薄膜表面及晶界处2D/3D分子连接示意图,SEM图像:(d) MAPbI3膜,(e) BA处理的MAPbI3膜,(f) BAI处理的MAPbI3膜[57]Fig. 4 Schematic figure of (a) the perovskite film treated by BA to form a 2D/3D stacking structure and (b,c) 2D/3D molecular junctions on the surface and at grain boundaries of 3D perovskite films induced by BA and BAI treatments, respectively; SEM images of (d) MAPbI3 films, (e) BA-treated MAPbI3 films, and (f) BAI-treated MAPbI3 films[57] (Reproduced with permission from ref 57) |
图5 (a) FAI和iBAI钝化处理方法示意图;(b)稳定性测试:在75% RH条件下38 d的PCEs监控;(c) 各种钝化组合物的PCE、VOC、JSC、FF分布[59]Fig. 5 (a) Schematic of the MP passivation treatment method with FAI and iBAI; (b) Stability test: PCEs monitored in 75% RH condition over a period of 38 d; (c) Distribution of PCE, VOC, JSC, and FF of devices with various passivation compositions[59] (Reproduced with permission from ref 59) |
图6 钙钛矿表面示意图(a) PEAI改性,其中PEA+离子似乎垂直竖立;(b) ODAI改性,ODA2+离子似乎水平放置(颜色:原子,红色:I,灰色:铅,蓝色:H,黄色:C,棕色:N);(c)在黑暗条件下,设备在湿度约为20% ~ 40%的环境中,其性能是存储时间的函数,插图显示了对照(左)和ODAI修改的设备(右)的最终照片[60]Fig. 6 Schematic of the surface of perovskite with (a) PEAI modification wherein the PEA+ ion seemed to stand vertically and (b) ODAI modification wherein the ODA2+ ion seemed to lie horizontally (color: atom, red: I, gray: Pb, blue: H, yellow: C and brown: N); (c) Device performance as a function of storage time in an ambient environment with a humidity of about 20%~40% under the dark condition, and the inset shows the final photographs of control (left) and ODAI-modified (right) devices[60] (Reproduced with permission from ref 60) |
图7 (a)具有吸湿性分子的分子结构,(b)具有路易斯碱基官能团的分子结构[62,64]Fig. 7 (a) Molecular structure of the molecules with hygroscopic molecules, (b) Molecular structure of the molecules with Lewis base functional groups[62,64] (Figure a is reproduced with permission from ref 62, Figure b is reproduced with permission from ref 64) |
图9 在黑暗潮湿的环境中,使用或不使用OA表面钝化的老化MAPbI3薄膜的紫外-可见吸收光谱;(a) MAPbI3膜老化前后1周的吸收光谱;(b) 使用OA的MAPbI3膜在老化前后1周和4周的吸收光谱,插图显示相应样品的图像[68]Fig. 9 UV-vis absorption spectra of aged MAPbI3 films with and without surface passivation with OA in a dark and humid environment; (a) The absorption spectra of MAPbI3 before and after 1 week of aging; (b) The absorption spectra of MAPbI3 film with OA before and after 1 and 4 weeks of aging, Insets show photographic images of the corresponding samples[68] (Reproduced with permission from ref 68) |
图12 (a) MAPbI3晶格末端处AVA钝化的示意图,MAPbI3表面缺陷位点经AVA钝化后稳定性增强的示意图;(b)在没有AVA的情况下,氧可以进入晶界的碘空位,从而导致在光辐射下超氧化物介导的光降解;(c)在有AVA存在的情况下,AVA与这些碘空位结合,抑制这种降解[71]Fig. 12 (a) Schematic representation of AVA passivation at lattice termination of MAPbI3, schematic representation of enhanced stability resulting from AVA passivation of surface defect sites of MAPbI3: in the absence of AVA (b), oxygen can access iodide vacancies at grain boundaries, resulting under irradiation in superoxide mediated photodegradation; in the presence of AVA (c), AVA binds to these iodide vacancies, inhibiting this degradation[71] (Reproduced with permission from ref 71) |
图14 (a)高湿度老化前后原始和钝化钙钛矿薄膜的照片;(b)新制备和老化钙钛矿薄膜的XRD图谱;(c)在室温相对湿度为60%~70%的环境中贮存的参考装置和2-MP钝化装置的稳定性试验[77]Fig. 14 (a) Photographs of the pristine and passivated perovskite films before and after high humidity aging; (b) XRD patterns of freshly prepared and aged perovskite films. (c) Stability test of the reference and 2-MP passivated devices stored in ambient air with a relative humidity of 60%~70% at room temperature[77] (Reproduced with permission from ref 77) |
表1 PSCs缺陷钝化概述:钝化剂、结构、钙钛矿材料、钝化功能基团、钝化类型(二维钝化)/靶缺陷(分子钝化)、无(C)和有(P)钝化的光伏参数Table 1 Summary of defect passivation for PSCs: passivator, structure, perovskite materials, passivation functional group, passivation type (two-dimensional passivation)/targeted defect (molecular passivation) and photovoltaic parameters without (C) and with (P) passivation (A: average; PVK: perovskite) |
| Passivator | Structure | Perovskite | Passivation functional group | Passivation type/ Targeted defects | Jsc[mA/ cm2] (C/P) | Voc[V] (C/P) | FF (C/P) | PCE [%] (C/P) | ref | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEAI | 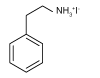 | MAPbI3 | Ammonium | 2D | 23.58/22.69 | 1.104/1.146 | 0.7685/0.7632 | 20.0/19.84 | 56 | |||||||||
| BA/BAI | 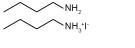 | MAPbI3 | Amine/Ammonium | 2D | 22.20/22.49、22.59 | 1.08/1.11, 1.09 | 0.74/0.78, 0.77 | 17.75/19.56、18.85 | 57 | |||||||||
| ZnPc |  | MAPbI3 | Ammonium | 2D | 22.93/23.23 | 1.08/1.09 | 0.76/0.77 | 18.83/19.56 | 58 | |||||||||
| ODAI | 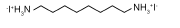 | FAPbI3 | Ammonium | 2D | 24.81/24.90 | 1.04/1.13 | 0.78/0.75 | 20.23/21.18 | 60 | |||||||||
| FPEAI | 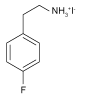 | Cs0.1(FA0.83 MA0.17)0.9 Pb(I0.83Br0.17)3 | Ammonium | 2D | 22.04/22.80 | 1.090/1.126 | 0.80/0.80 | 19.22/20.54 | 61 | |||||||||
| BA | 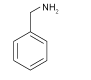 | FAPbI3 | Amine | Undercoor- dinated Pb2+ or the iodide ions | 22.7/23.6 | 1.01/1.12 | 0.70/0.73 | 15.7/19.2 | 65 | |||||||||
| PVP |  | MAPbI3 | N donor (pyridine group) | Undercoordinated Pb2+ | 20.1/22.0 | 0.90/1.05 | 0.64/0.66 | 11.6/15.1 | 66 | |||||||||
| PEO | 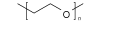 | MAPbI3 | O donor | Undercoordinated Pb2+ | 19.823/20.850 | 1.055/1.105 | 0.750/0.754 | 15.552/17.194 | 67 | |||||||||
| OA | 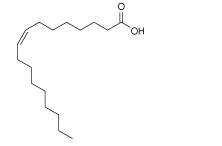 | MAPbI3 | Carboxyl group | Surface Pb2+ and/or CH3N | 24.4/23.5 | 0.86/0.93 | 36.0/41.7 | 7.62/9.11 | 68 | |||||||||
| Passivator | Structure | Perovskite | Passivation functional group | Passivation type/ Targeted defects | Jsc[mA/ cm2] (C/P) | Voc[V] (C/P) | FF (C/P) | PCE [%] (C/P) | ref | |||||||||
| PCDTBT | 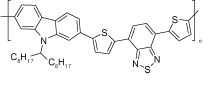 | CH3NH3 PbIxCl3-x | S, N donor | Undercoordinated Pb2+ | 20.87/21.71 | 0.91/0.94 | 0.69/0.77 | 13.19/15.76 | 69 | |||||||||
| BAA |  | Cs/FA/MA PVK MAPbI3 | Amine | Undercoordinated Pb2+ | 23.4/23.4 22.0/22.5 | 1.06/1.16 1.08/1.18 | 0.684/0.794 0.772/0.817 | 17.0/21.5 18.3/21.7 | 70 | |||||||||
| PBDB-T | 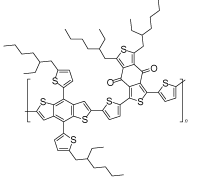 | (CsPbI3)0.04 (FAPbI3)0.82 (MAPbBr3)0.14 | O donor | Undercoordinated Pb2+ | 21.73/22.39 | 1.075/1.113 | 0.740/0.778 | 17.28/19.38 | 72 | |||||||||
| AQ310 | 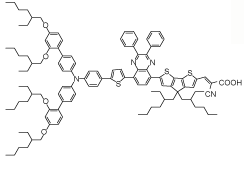 | (FAPbI3)0.85 (MAPbBr3)0.15 | Carboxyl group | Undercoordinated Pb2+ | 21.76/21.80 | 1.11/1.15 | 0.780/0.784 | 18.84(17.98 A)/19.66(19.43 A) | 73 | |||||||||
| LL |  | MAPbI3 | Bipolarity | Anionic defects | 21.35/24.09 | 1.00/1.02 | 0.728/0.741 | 15.55/18.20 | 74 | |||||||||
| FAL | 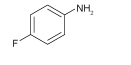 | Cs0.05(MA0.17 FA0.83)0.95 Pb(I0.83Br0.17)3 | Amine | The sites of MA/FA vacancies | 22.56/23.33 | 1.02/1.33 | 0.743/0.777 | 17.08/20.48 | 75 | |||||||||
| 2-MP | 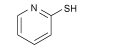 | MAPbI3 | N donor (pyridine ring) and S donor | Undercoordinated Pb2+ | 22.56/22.61 | 1.09/1.16 | 0.7464/0.7744 | 18.35/20.28 | 77 | |||||||||
| HS | 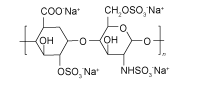 | MAPbI3 | the-COO-/-S anionic and Na+ cationic groups | Undersaturated Pb2+ and I- in MAPbI3 and Ti4+ in TiO2 | 21.29/23.34 | 1.090/1.114 | 0.7407/0.7731 | 17.20/20.10 | 78 | |||||||||
| [1] |
Chen S, Shi G. Adv. Mater., 2017,29:1605448.
|
| [2] |
Yan J L, Qiu W M, Wu G, Heremans P, Chen H Z . J. Mater. Chem. A, 2018,6:11063.
|
| [3] |
Xing G C, Mathews N, Sun S Y, Lim S S, Lam Y M, Gratzel M, Mhaisalkar S, Sum T C . Science, 2013,342:344. https://www.ncbi.nlm.nih.gov/pubmed/24136965
DOI: 10.1126/science.1243167 PMID: 24136965 Low-temperature solution-processed photovoltaics suffer from low efficiencies because of poor exciton or electron-hole diffusion lengths (typically about 10 nanometers). Recent reports of highly efficient CH3NH3PbI3-based solar cells in a broad range of configurations raise a compelling case for understanding the fundamental photophysical mechanisms in these materials. By applying femtosecond transient optical spectroscopy to bilayers that interface this perovskite with either selective-electron or selective-hole extraction materials, we have uncovered concrete evidence of balanced long-range electron-hole diffusion lengths of at least 100 nanometers in solution-processed CH3NH3PbI3. The high photoconversion efficiencies of these systems stem from the comparable optical absorption length and charge-carrier diffusion lengths, transcending the traditional constraints of solution-processed semiconductors. |
| [4] |
Lin Q Q, Armin A, Burn P L, Meredith P . Nature Photonics, 2015,9:687.
|
| [5] |
Marchioro A, Teuscher J, Friedrich D, Kunst M, van de Krol R, Moehl T, Gratzel M, Moser J E. Nature Photonics, 2014,8:250.
|
| [6] |
Protesescu L, Yakunin S, Bodnarchuk M I, Krieg F, Caputo R, Hendon C H, Yang R X, Walsh A, Kovalenko M V. Nano Lett., 2015,15:3692. https://www.ncbi.nlm.nih.gov/pubmed/25633588
DOI: 10.1021/nl5048779 PMID: 25633588 Metal halides perovskites, such as hybrid organic-inorganic CH3NH3PbI3, are newcomer optoelectronic materials that have attracted enormous attention as solution-deposited absorbing layers in solar cells with power conversion efficiencies reaching 20%. Herein we demonstrate a new avenue for halide perovskites by designing highly luminescent perovskite-based colloidal quantum dot materials. We have synthesized monodisperse colloidal nanocubes (4-15 nm edge lengths) of fully inorganic cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I or mixed halide systems Cl/Br and Br/I) using inexpensive commercial precursors. Through compositional modulations and quantum size-effects, the bandgap energies and emission spectra are readily tunable over the entire visible spectral region of 410-700 nm. The photoluminescence of CsPbX3 nanocrystals is characterized by narrow emission line-widths of 12-42 nm, wide color gamut covering up to 140% of the NTSC color standard, high quantum yields of up to 90%, and radiative lifetimes in the range of 1-29 ns. The compelling combination of enhanced optical properties and chemical robustness makes CsPbX3 nanocrystals appealing for optoelectronic applications, particularly for blue and green spectral regions (410-530 nm), where typical metal chalcogenide-based quantum dots suffer from photodegradation. |
| [7] |
Kojima A, Teshima K, Shirai Y, Miyasaka T . J. Am. Chem. Soc., 2009,131:6050. https://www.ncbi.nlm.nih.gov/pubmed/19366264
DOI: 10.1021/ja809598r PMID: 19366264 Two organolead halide perovskite nanocrystals, CH(3)NH(3)PbBr(3) and CH(3)NH(3)PbI(3), were found to efficiently sensitize TiO(2) for visible-light conversion in photoelectrochemical cells. When self-assembled on mesoporous TiO(2) films, the nanocrystalline perovskites exhibit strong band-gap absorptions as semiconductors. The CH(3)NH(3)PbI(3)-based photocell with spectral sensitivity of up to 800 nm yielded a solar energy conversion efficiency of 3.8%. The CH(3)NH(3)PbBr(3)-based cell showed a high photovoltage of 0.96 V with an external quantum conversion efficiency of 65%. |
| [8] |
Zimmermann I, Aghazada S, Nazeeruddin M K. Angew. Chem. Int. Ed., 2019,58:1072. https://www.ncbi.nlm.nih.gov/pubmed/30462878
DOI: 10.1002/anie.201811497 PMID: 30462878 3+ amino groups, with optical band gap values of 1.81 eV and 1.79 eV for Bn2 SnI4 and BdiSnI4 respectively. Furthermore, we demonstrate that the optical properties in this class of perovskites can be tuned by formation of a quasi 2D perovskite with the formula Bn2 FASn2 I7 . Additionally, we investigate the change in band gap in the mixed Sn/Pb solid solution Bn2 Snx Pbx-1 I4 . Devices fabricated with Bn2 SnI4 show promising efficiencies of around 2.3 %.]]> |
| [9] |
Lee M M, Teuscher J, Miyasaka T, Murakami T N, Snaith H J . Science, 2012,338:643. https://www.ncbi.nlm.nih.gov/pubmed/23042296
DOI: 10.1126/science.1228604 PMID: 23042296 The energy costs associated with separating tightly bound excitons (photoinduced electron-hole pairs) and extracting free charges from highly disordered low-mobility networks represent fundamental losses for many low-cost photovoltaic technologies. We report a low-cost, solution-processable solar cell, based on a highly crystalline perovskite absorber with intense visible to near-infrared absorptivity, that has a power conversion efficiency of 10.9% in a single-junction device under simulated full sunlight. This "meso-superstructured solar cell" exhibits exceptionally few fundamental energy losses; it can generate open-circuit photovoltages of more than 1.1 volts, despite the relatively narrow absorber band gap of 1.55 electron volts. The functionality arises from the use of mesoporous alumina as an inert scaffold that structures the absorber and forces electrons to reside in and be transported through the perovskite. |
| [10] |
Yang W S, Park B W, Jung E H, Jeon N J, Kim Y C, Lee D U, Shin S S, Seo J, Kim E K, Noh J H, Seok S I . Science, 2017,356:1376. https://www.ncbi.nlm.nih.gov/pubmed/28663498
DOI: 10.1126/science.aan2301 PMID: 28663498 The formation of a dense and uniform thin layer on the substrates is crucial for the fabrication of high-performance perovskite solar cells (PSCs) containing formamidinium with multiple cations and mixed halide anions. The concentration of defect states, which reduce a cell's performance by decreasing the open-circuit voltage and short-circuit current density, needs to be as low as possible. We show that the introduction of additional iodide ions into the organic cation solution, which are used to form the perovskite layers through an intramolecular exchanging process, decreases the concentration of deep-level defects. The defect-engineered thin perovskite layers enable the fabrication of PSCs with a certified power conversion efficiency of 22.1% in small cells and 19.7% in 1-square-centimeter cells. |
| [11] |
Jeon N J, Lee J, Noh J H, Nazeeruddin M K, Gratzel M, Seok S I . J. Am. Chem. Soc., 2013,135:19087. https://www.ncbi.nlm.nih.gov/pubmed/24313292
DOI: 10.1021/ja410659k PMID: 24313292 A set of three N,N-di-p-methoxyphenylamine-substituted pyrene derivatives have successfully been synthesized and characterized by (1)H/(13)C NMR spectroscopy, mass spectrometry, and elemental analysis. The optical and electronic structures of the pyrene derivatives were adjusted by controlling the ratio of N,N-di-p-methoxyphenylamine to pyrene, and investigated by UV/vis spectroscopy and cyclic voltammetry. The pyrene derivatives were employed as hole-transporting materials (HTMs) in fabricating mesoporous TiO2/CH3NH3PbI3/HTMs/Au solar cells. The pyrene-based derivative Py-C exhibited a short-circuit current density of 20.2 mA/cm(2), an open-circuit voltage (Voc) of 0.886 V, and a fill factor of 69.4% under an illumination of 1 sun (100 mW/cm(2)), resulting in an overall power conversion efficiency of 12.4%. The performance is comparable to that of the well-studied spiro-OMeTAD, even though the Voc is slightly lower. Thus, this newly synthesized pyrene derivative holds promise as a HTM for highly efficient perovskite-based solar cells. |
| [12] |
Jeon N J, Na H, Jung E H, Yang T Y, Lee Y G, Kim G, Shin H W, Seok S I, Lee J, Seo J . Nature Energy, 2018,3:682.
|
| [13] |
NREL Efficiency Chart Vol. 2019. [2019-05-30]https://www.nrel.gov/pv/cell-efficiency.html. https://www.nrel.gov/pv/cell-efficiency.html
|
| [14] |
Shi D, Adinolfi V, Comin R, Yuan M, Alarousu E, Buin A, Chen Y, Hoogland S, Rothenberger A, Katsiev K, Losovyj Y, Zhang X, Dowben P A, Mohammed O F, Sargent E H, Bakr O M . Science, 2015,347:519. https://www.ncbi.nlm.nih.gov/pubmed/25635092
DOI: 10.1126/science.aaa2725 PMID: 25635092 The fundamental properties and ultimate performance limits of organolead trihalide MAPbX3 (MA = CH3NH3(+); X = Br(-) or I(-)) perovskites remain obscured by extensive disorder in polycrystalline MAPbX3 films. We report an antisolvent vapor-assisted crystallization approach that enables us to create sizable crack-free MAPbX3 single crystals with volumes exceeding 100 cubic millimeters. These large single crystals enabled a detailed characterization of their optical and charge transport characteristics. We observed exceptionally low trap-state densities on the order of 10(9) to 10(10) per cubic centimeter in MAPbX3 single crystals (comparable to the best photovoltaic-quality silicon) and charge carrier diffusion lengths exceeding 10 micrometers. These results were validated with density functional theory calculations. |
| [15] |
Dong Q, Fang Y, Shao Y, Mulligan P, Qiu J, Cao L, Huang J . Science, 2015,347:967. https://www.ncbi.nlm.nih.gov/pubmed/25636799
DOI: 10.1126/science.aaa5760 PMID: 25636799 Long, balanced electron and hole diffusion lengths greater than 100 nanometers in the polycrystalline organolead trihalide compound CH3NH3PbI3 are critical for highly efficient perovskite solar cells. We found that the diffusion lengths in CH3NH3PbI3 single crystals grown by a solution-growth method can exceed 175 micrometers under 1 sun (100 mW cm(-2)) illumination and exceed 3 millimeters under weak light for both electrons and holes. The internal quantum efficiencies approach 100% in 3-millimeter-thick single-crystal perovskite solar cells under weak light. These long diffusion lengths result from greater carrier mobility, longer lifetime, and much smaller trap densities in the single crystals than in polycrystalline thin films. The long carrier diffusion lengths enabled the use of CH3NH3PbI3 in radiation sensing and energy harvesting through the gammavoltaic effect, with an efficiency of 3.9% measured with an intense cesium-137 source. |
| [16] |
Shao Y, Fang Y, Li T, Wang Q, Dong Q, Deng Y, Yuan Y, Wei H, Wang M, Gruverman A, Shield J, Huang J. Energy Environ. Sci., 2016,9:1752.
|
| [17] |
Zong Y, Zhou Y, Zhang Y, Li Z, Zhang L, Ju M, Chen M, Pang S, Zeng X C, Padture N P . Chem., 2018,4:1404.
|
| [18] |
Deng W, Liang X, Kubiak P S, Cameron P J. Adv. Energy Mater., 2018,8:1701544.
|
| [19] |
Li W, Zhang C, Ma Y, Liu C, Fan J, Mai Y, Schropp R E I . Energy Environ. Sci., 2018,11:286. http://xlink.rsc.org/?DOI=C7EE03113K
DOI: 10.1039/C7EE03113K |
| [20] |
张佳维(Zhang J W) . 山东化工(Shandong Chemical Industry), 2018,47:66.
|
| [21] |
Correa-Baena J P, Saliba M, Buonassisi T, Gratzel M, Abate A, Tress W, Hagfeldt A . Science, 2017,358:739. https://www.ncbi.nlm.nih.gov/pubmed/29123060
DOI: 10.1126/science.aam6323 PMID: 29123060 The efficiencies of perovskite solar cells have gone from single digits to a certified 22.1% in a few years' time. At this stage of their development, the key issues concern how to achieve further improvements in efficiency and long-term stability. We review recent developments in the quest to improve the current state of the art. Because photocurrents are near the theoretical maximum, our focus is on efforts to increase open-circuit voltage by means of improving charge-selective contacts and charge carrier lifetimes in perovskites via processes such as ion tailoring. The challenges associated with long-term perovskite solar cell device stability include the role of testing protocols, ionic movement affecting performance metrics over extended periods of time, and determination of the best ways to counteract degradation mechanisms. |
| [22] |
Kieslich G, Sun S J, Cheetham A K. Chem. Sci., 2014,5:4712.
|
| [23] |
Grancini G, Nazeeruddin M.K. Dimensional tailoring of hybrid perovskites for photovoltaics. Nature Reviews Materials, 2019. 4:4.
|
| [24] |
a) Conings B, Drijkoningen J, Gauquelin N, Babayigit A, D’Haen J, D’Olieslaeger L, Ethirajan A, Verbeeck J, Manca J, Mosconi E, Angelis F D, Boyen H G . Adv. Energy Mater., 2015. 5:1500477;
b) Dualeh A, Gao P, Seok S I, Nazeeruddin M K, Grätzel M. Chem. Mater., 2014. 26:6160; https://www.ncbi.nlm.nih.gov/pubmed/27109865
DOI: 10.1021/acs.jpcb.6b02165 PMID: 27109865 We explore the electrophoretic propagation of charged colloidal objects, monodisperse anionically stabilized polystyrene spheres, in large-pore agarose gels that have been passivated using polyethylene glycol (PEG) when a radial electric field is applied in a cylindrical geometry. By contrast to standard Cartesian gel-electrophoresis geometries, in a cylindrical geometry, charged particles that start at a ring well near the central axis propagate outward more rapidly initially and then slow down as they move further away from the axis. By building a full-ring cylindrical gel electrophoresis chamber and taking movies of scattered light from propagating nanospheres undergoing electrophoresis, we experimentally demonstrate that the ring-like front of monodisperse nanospheres propagates stably in PEG-passivated agarose gels and that the measured ring radius as a function of time agrees with a simple model that incorporates the electric field of a cylindrical geometry. Moreover, we show that this cylindrical geometry offers a potential advantage when performing electrophoretic separations of objects that have widely different sizes: smaller objects can still be retained in a cylindrical gel that has a limited size over long electrophoretic run times required for separating larger objects.
c) Lee S W, Kim S, Bae S, Cho K, Chung T, Mundt L E, Lee S, Park S, Park H, Schubert M C, Glunz S W, Ko Y, Jun Y, Kang Y, Lee H S, Kim D. Sci. Rep., 2016. 6:38150; https://www.ncbi.nlm.nih.gov/pubmed/27909338
DOI: 10.1038/srep38150 PMID: 27909338 sc) and EQE continuously restored. 1-sun light soaking induced recovery is considered to be caused by resolving of stacked charges and defect state neutralization. The Jsc and EQE bounce-back phenomenon is attributed to the beneficial effects of PbI2 which is generated by the decomposition of perovskite material.]]>
d) Niu G, Guo X, Wang L. J. Mater. Chem. A., 2015. 3:8970.
|
| [25] |
a) Han Y, Meyer S, Dkhissi Y, Weber K, Pringle J M, Bach U, Spiccia L, Cheng Y B . J. Mater. Chem. A, 2015. 3:8139;
b) Leguy A M A, Hu Y, Campoy-Quiles M, Alonso M I, Weber O J, Azarhoosh P, van Schilfgaarde M, Weller M T, Bein T, Nelson J, Docampo P, Barnes P R F. Chem. Mater., 2015. 27:3397; https://www.ncbi.nlm.nih.gov/pubmed/32260930
DOI: 10.1039/c3tb20386g PMID: 32260930 Antifouling and antibacterial membranes are prepared by selective surface modification of pH responsive polystyrene-b-poly(4-vinylpyridine) (PS-P4VP) diblock copolymers by quaternization and zwitterionization reactions on a P4VP moiety. Nanoporous membranes based on the self-assembly of 2-(4'-hydroxybenzeneazo) benzoic acid (HABA)-PS-P4VP supramolecular complexes and nonsolvent induced phase separation are first prepared and the surfaces are functionalized by crosslinking with diiodobutane vapors and reacting with propane sultone vapors at moderate temperature and under vacuum conditions. Selective functionalization of surfaces is carried out to enhance the antifouling and antibiofouling properties of the membrane and to retain its pH switching behavior. The membranes are thoroughly characterized by various instrumental techniques such as Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscopy, quartz crystal microbalance, contact angle, etc. Antifouling and antibacterial properties are proven by analyzing the adsorption of bovine serum albumin protein and bacterial cell attachment and killing efficiency. The actual membrane performance is assessed in terms of water flux under different pressures and pHs and Congo red dye rejection efficiency.
c) Zhang L, Sit P H L. J. Phys. Chem. C, 2015,119:22370.
|
| [26] |
Soufiani A M, Hameiri Z, Meyer S, Lim S, Tayebjee M J Y, Yun J S, Ho-Baillie A, Conibeer G J, Spiccia L, Green M A . Adv. Energy Mater., 2017,7:1602111.
|
| [27] |
Wang D, Wright M, Elumalai N K, Uddin A. Sol. Energy Mater. Sol. Cells, 2016,147:255.
|
| [28] |
Berhe T A, Su W N, Chen C H, Pan C J, Cheng J H, Chen H M, Tsai M C, Chen L Y, Dubale A A, Hwang B J. Energy Environ. Sci., 2016,9:323.
|
| [29] |
Aristidou N, Eames C, Sanchez-Molina I, Bu X N, Kosco J, Islam M S, Haque S A . Nature Communications, 2017,8:15218. https://www.ncbi.nlm.nih.gov/pubmed/28492235
DOI: 10.1038/ncomms15218 PMID: 28492235 3NH3PbI3 undergo rapid degradation when exposed to oxygen and light. Here, we report mechanistic insights into this oxygen-induced photodegradation from a range of experimental and computational techniques. We find fast oxygen diffusion into CH3NH3PbI3 films is accompanied by photo-induced formation of highly reactive superoxide species. Perovskite films composed of small crystallites show higher yields of superoxide and lower stability. Ab initio simulations indicate that iodide vacancies are the preferred sites in mediating the photo-induced formation of superoxide species from oxygen. Thin-film passivation with iodide salts is shown to enhance film and device stability. The understanding of degradation phenomena gained from this study is important for the future design and optimization of stable perovskite solar cells.]]> |
| [30] |
Qiu W M, Ray A, Jaysankar M, Merckx T, Bastos J P, Cheyns D, Gehlhaar R, Poortmans J, Heremans P . Adv. Funct. Mater., 2017,27:1700920.
|
| [31] |
Saliba M, Matsui T, Seo J Y, Domanski K Correa-Baena J P, Nazeeruddin M K, Zakeeruddin S M, Tress W, Abate A, Hagfeldt A, Gratzel M . Energy Environ. Sci., 2016,9:1989. https://www.ncbi.nlm.nih.gov/pubmed/27478500
DOI: 10.1039/c5ee03874j PMID: 27478500 Today's best perovskite solar cells use a mixture of formamidinium and methylammonium as the monovalent cations. With the addition of inorganic cesium, the resulting triple cation perovskite compositions are thermally more stable, contain less phase impurities and are less sensitive to processing conditions. This enables more reproducible device performances to reach a stabilized power output of 21.1% and ∼18% after 250 hours under operational conditions. These properties are key for the industrialization of perovskite photovoltaics. |
| [32] |
Li F, Wang H, Kufer D, Liang L L, Yu W L, Alarousu E, Ma C, Li Y Y, Liu Z X, Liu C X, Wei N N, Wang F, Chen L, Mohammed O F, Fratalocchi A, Liu X G, Konstantatos G, Wu T. Adv. Mater., 2017,29:1602432.
|
| [33] |
Chen H L, Fu W F, Huang C Y, Zhang Z Q, Li S X, Ding F Z, Shi M M, Li C Z, Jen A K Y, Chen H Z . Adv. Energy Mater., 2017,7:1700012.
|
| [34] |
Dong Q, Liu F Z, Wong M K, Tam H W, Djurisic A B, Ng A N, Surya C, Chan W K, Ng A M C . ChemSusChem, 2016,9:2597. https://www.ncbi.nlm.nih.gov/pubmed/27504719
DOI: 10.1002/cssc.201600868 PMID: 27504719 2 film and encapsulated with a UV-curable epoxy including a desiccant sheet. However, the stability of ZnO-based cells encapsulated by the same method was found to be inferior to that of TiO2 -based cells. Finally, outdoor performance tests were performed for TiO2 -based cells (30-90 % ambient humidity). All the stability tests were performed following the established international summit on organic photovoltaic stability (ISOS) protocols for organic solar cell testing (ISOS-L2 and ISOS-O1).]]> |
| [35] |
Brinkmann K O, Zhao J, Pourdavoud N, Becker T, Hu T, Olthof S, Meerholz K, Hoffmann L, Gahlmann T, Heiderhoff R, Oszajca M F, Luechinger N A, Rogalla D, Chen Y, Cheng B, Riedl T . Nature Communications, 2017,8:13938. https://www.ncbi.nlm.nih.gov/pubmed/28067308
DOI: 10.1038/ncomms13938 PMID: 28067308 The area of thin-film photovoltaics has been overwhelmed by organometal halide perovskites. Unfortunately, serious stability concerns arise with perovskite solar cells. For example, methyl-ammonium lead iodide is known to decompose in the presence of water and, more severely, even under inert conditions at elevated temperatures. Here, we demonstrate inverted perovskite solar cells, in which the decomposition of the perovskite is significantly mitigated even at elevated temperatures. Specifically, we introduce a bilayered electron-extraction interlayer consisting of aluminium-doped zinc oxide and tin oxide. We evidence tin oxide grown by atomic layer deposition does form an outstandingly dense gas permeation barrier that effectively hinders the ingress of moisture towards the perovskite and-more importantly-it prevents the egress of decomposition products of the perovskite. Thereby, the overall decomposition of the perovskite is significantly suppressed, leading to an outstanding device stability. |
| [36] |
Koushik D, Verhees W J H, Kuang Y H, Veenstra S, Zhang D, Verheijen M A, Creatore M, Schropp R E I . Energy Environ. Sci., 2016,10:91.
|
| [37] |
Xiang W C, Chen Q, Wang Y Y, Liu M J, Huang F Z, Bu T L, Wang T S, Cheng Y B, Gong X, Zhong J, Liu P, Yao X, Zhao X J . J. Mater. Chem. A, 2017,5:5486.
|
| [38] |
Gao P, Cho K T, Abate A, Grancini G, Reddy P Y, Srivasu M, Adachi M, Suzuki A, Tsuchimoto K, Gratzel M, Nazeeruddin M K. Phys. Chem. Chem. Phys., 2016,18:27083. https://www.ncbi.nlm.nih.gov/pubmed/27400647
DOI: 10.1039/c6cp03396b PMID: 27400647 2O3 for the infiltration of sym-HTPcH to form a hybrid interfacial buffer layer, affording perovskite solar cells (PSCs) with an average PCE value of up to 12.3%, which is a significant improvement with respect to the control cell without the meso-Al2O3 layer (4.21%) and is the highest value ever reported for Zn(ii) phthalocyanine based devices under AM1.5G standard conditions. A hysteresis test revealed that our device structure with the new HTM exhibited a balanced charge extraction behaviour.]]> |
| [39] |
Nam J K, Chai S U, Cha W, Choi Y J, Kim W, Jung M S, Kwon J, Kim D, Park H . Nano Lett., 2017,17:2028. https://www.ncbi.nlm.nih.gov/pubmed/28170276
DOI: 10.1021/acs.nanolett.7b00050 PMID: 28170276 0.925K0.075PbI2Br, the planar-architecture device achieves a power conversion efficiency of 10.0%, which is a remarkable record in the field of inorganic perovskite solar cells. In addition, the device shows an extended operational lifetime against air. Our research will stimulate the development of cesium lead halide perovskite materials for next-generation photovoltaics.]]> |
| [40] |
Du M H . J. Mater. Chem. A, 2014,2:9091.
|
| [41] |
Arora N, Dar M I, Hinderhofer A, Pellet N, Schreiber F, Zakeeruddin S M, Gratzel M . Science, 2017,358:768. https://www.ncbi.nlm.nih.gov/pubmed/28971968
DOI: 10.1126/science.aam5655 PMID: 28971968 Perovskite solar cells (PSCs) with efficiencies greater than 20% have been realized only with expensive organic hole-transporting materials. We demonstrate PSCs that achieve stabilized efficiencies exceeding 20% with copper(I) thiocyanate (CuSCN) as the hole extraction layer. A fast solvent removal method enabled the creation of compact, highly conformal CuSCN layers that facilitate rapid carrier extraction and collection. The PSCs showed high thermal stability under long-term heating, although their operational stability was poor. This instability originated from potential-induced degradation of the CuSCN/Au contact. The addition of a conductive reduced graphene oxide spacer layer between CuSCN and gold allowed PSCs to retain >95% of their initial efficiency after aging at a maximum power point for 1000 hours under full solar intensity at 60°C. Under both continuous full-sun illumination and thermal stress, CuSCN-based devices surpassed the stability of spiro-OMeTAD-based PSCs. |
| [42] |
Zhang J J, Zhang L Y, Li X H, Zhu X Y, Yu J G, Fan K. ACS Sustainable Chem. Eng., 2019,7:3487. https://pubs.acs.org/doi/10.1021/acssuschemeng.8b05734
|
| [43] |
Gao P, Yusoff A B, Nazeeruddin M K . Nature Communications, 2018. 9:5028. https://www.ncbi.nlm.nih.gov/pubmed/30487520
DOI: 10.1038/s41467-018-07382-9 PMID: 30487520 Hybrid halide perovskite solar cells were first demonstrated in 2009 with cell efficiency quickly soaring from below 10% to more than 23% in a few years. Halide perovskites have the desirable processing simplicity but are very fragile when exposed to water and heat. This fragility represents a great challenge for the achievement of their full practical potential in photovoltaic technologies. To address this problem, here we review the recent development of the mixed-dimensional perovskites, whereby the trade-off between power conversion efficiency and stability of the material can be finely tuned using organic amine cations with different sizes and functionalities. |
| [44] |
Yang Y, Yang M, Moore D T, Yan Y, Miller E M, Zhu K, Beard M C . Nat. Energy, 2017,2:16207.
|
| [45] |
Shao Y, Xiao Z, Bi C, Yuan Y, Huang J . Nat. Commun., 2014,5:5784. https://www.ncbi.nlm.nih.gov/pubmed/25503258
DOI: 10.1038/ncomms6784 PMID: 25503258 The large photocurrent hysteresis observed in many organometal trihalide perovskite solar cells has become a major hindrance impairing the ultimate performance and stability of these devices, while its origin was unknown. Here we demonstrate the trap states on the surface and grain boundaries of the perovskite materials to be the origin of photocurrent hysteresis and that the fullerene layers deposited on perovskites can effectively passivate these charge trap states and eliminate the notorious photocurrent hysteresis. Fullerenes deposited on the top of the perovskites reduce the trap density by two orders of magnitude and double the power conversion efficiency of CH(3)NH(3)PbI(3) solar cells. The elucidation of the origin of photocurrent hysteresis and its elimination by trap passivation in perovskite solar cells provides important directions for future enhancements to device efficiency. |
| [46] |
Xu J, Buin A, Ip A H, Li W, Voznyy O, Comin R, Yuan M, Jeon S, Ning Z, McDowell J J, Kanjanaboos P, Sun J P, Lan X, Quan L N, Kim D H, Hill I G, Maksymovych P, Sargent E H . Nat. Commun., 2015,6:7081. https://www.ncbi.nlm.nih.gov/pubmed/25953105
DOI: 10.1038/ncomms8081 PMID: 25953105 Solution-processed planar perovskite devices are highly desirable in a wide variety of optoelectronic applications; however, they are prone to hysteresis and current instabilities. Here we report the first perovskite-PCBM hybrid solid with significantly reduced hysteresis and recombination loss achieved in a single step. This new material displays an efficient electrically coupled microstructure: PCBM is homogeneously distributed throughout the film at perovskite grain boundaries. The PCBM passivates the key PbI3(-) antisite defects during the perovskite self-assembly, as revealed by theory and experiment. Photoluminescence transient spectroscopy proves that the PCBM phase promotes electron extraction. We showcase this mixed material in planar solar cells that feature low hysteresis and enhanced photovoltage. Using conductive AFM studies, we reveal the memristive properties of perovskite films. We close by positing that PCBM, by tying up both halide-rich antisites and unincorporated halides, reduces electric field-induced anion migration that may give rise to hysteresis and unstable diode behaviour. |
| [47] |
Wang Q, Shao Y, Dong Q, Xiao Z, Yuan Y, Huang J. Energy Environ. Sci., 2014,7:2359.
|
| [48] |
De Marco N, Zhou H, Chen Q, Sun P, Liu Z, Meng L, Yao E P, Liu Y, Schiffer A, Yang Y . Nano Lett., 2016,16:1009. https://www.ncbi.nlm.nih.gov/pubmed/26790037
DOI: 10.1021/acs.nanolett.5b04060 PMID: 26790037 Hybrid perovskites have shown astonishing power conversion efficiencies owed to their remarkable absorber characteristics including long carrier lifetimes, and a relatively substantial defect tolerance for solution-processed polycrystalline films. However, nonradiative charge carrier recombination at grain boundaries limits open circuit voltages and consequent performance improvements of perovskite solar cells. Here we address such recombination pathways and demonstrate a passivation effect through guanidinium-based additives to achieve extraordinarily enhanced carrier lifetimes and higher obtainable open circuit voltages. Time-resolved photoluminescence measurements yield carrier lifetimes in guanidinium-based films an order of magnitude greater than pure-methylammonium counterparts, giving rise to higher device open circuit voltages and power conversion efficiencies exceeding 17%. A reduction in defect activation energy of over 30% calculated via admittance spectroscopy and confocal fluorescence intensity mapping indicates successful passivation of recombination/trap centers at grain boundaries. We speculate that guanidinium ions serve to suppress formation of iodide vacancies and passivate under-coordinated iodine species at grain boundaries and within the bulk through their hydrogen bonding capability. These results present a simple method for suppressing nonradiative carrier loss in hybrid perovskites to further improve performances toward highly efficient solar cells. |
| [49] |
Chen Q, Zhou H, Song T B, Luo S, Hong Z, Duan H S, Dou L, Liu Y, Yang Y . Nano Lett., 2014,14:4158. https://www.ncbi.nlm.nih.gov/pubmed/24960309
DOI: 10.1021/nl501838y PMID: 24960309 To improve the performance of the polycrystalline thin film devices, it requires a delicate control of its grain structures. As one of the most promising candidates among current thin film photovoltaic techniques, the organic/inorganic hybrid perovskites generally inherit polycrystalline nature and exhibit compositional/structural dependence in regard to their optoelectronic properties. Here, we demonstrate a controllable passivation technique for perovskite films, which enables their compositional change, and allows substantial enhancement in corresponding device performance. By releasing the organic species during annealing, PbI2 phase is presented in perovskite grain boundaries and at the relevant interfaces. The consequent passivation effects and underlying mechanisms are investigated with complementary characterizations, including scanning electron microscopy (SEM), X-ray diffraction (XRD), time-resolved photoluminescence decay (TRPL), scanning Kelvin probe microscopy (SKPM), and ultraviolet photoemission spectroscopy (UPS). This controllable self-induced passivation technique represents an important step to understand the polycrystalline nature of hybrid perovskite thin films and contributes to the development of perovskite solar cells judiciously. |
| [50] |
Li X, Dar M I, Yi C, Luo J, Tschumi M, Zakeeruddin S M, Nazeeruddin M K, Han H, Gratzel M . Nat. Chem., 2015,7:703. https://www.ncbi.nlm.nih.gov/pubmed/26291941
DOI: 10.1038/nchem.2324 PMID: 26291941 In the past few years, organic-inorganic halide perovskites have rapidly emerged as promising materials for photovoltaic applications, but simultaneously achieving high performance and long-term stability has proved challenging. Here, we show a one-step solution-processing strategy using phosphonic acid ammonium additives that results in efficient perovskite solar cells with enhanced stability. We modify the surface of methylammonium lead triiodide (CH3NH3PbI3) perovskite by spin-coating its precursor solution in the presence of butylphosphonic acid 4-ammonium chloride. Morphological, structural and elemental analyses show that the phosphonic acid ammonium additive acts as a crosslink between neighbouring grains in the perovskite structure, through strong hydrogen bonding of the -PO(OH)2 and -NH3(+) terminal groups to the perovskite surface. The additives facilitate the incorporation of the perovskite within a mesoporous TiO2 scaffold, as well as the growth of a uniform perovskite layer at the surface, enhancing the material's photovoltaic performance from 8.8 to 16.7% as well as its resistance to moisture. |
| [51] |
Wang Q, Chen B, Liu Y, Deng Y, Bai Y, Dong Q, Huang J. Energy Environ. Sci., 2017,10:516.
|
| [52] |
Ahmad S, Guo X. Chinese Chemical Letters, 2018,29:657.
|
| [53] |
Mao L L, Tsai H, Nie W Y, Ma L, Im J, Stoumpos C C, Malliakas C D, Hao F, Wasielewski M R, Mohite A D, Kanatzidis M G. Chem. Mater., 2016,28:7781.
|
| [54] |
Abdel-Aal S K, Abdel-Rahman A S . J. Cryst. Growth, 2017,457:282.
|
| [55] |
Mercier N, Riou A . Chem. Commun., 2004,33:844.
|
| [56] |
Chen J, Lee D, Park N G. ACS Appl. Mater. Interfaces, 2017,9:36338. https://www.ncbi.nlm.nih.gov/pubmed/28952714
DOI: 10.1021/acsami.7b07595 PMID: 28952714 2PbI4 [PEA = C6H5(CH2)2NH3] in three-dimensional (3D) MAPbI3 [MA = CH3NH3 ] [denoted as (PEA2PbI4)x(MAPbI3)], where the perovskite films were fabricated by the Lewis acid-base adduct method. A nanolaminate structure comprising layered MAPbI3 nanobricks was created in the presence of 2D PEA2PbI4. For x = 0.017, a power conversion efficiency (PCE) of as high as 19.8% was achieved, which was comparable to the 20.0% PCE of a MAPbI3-based cell. Density functional theory (DFT) calculations confirmed that iodide migration was suppressed in the presence of the 2D perovskite as a result of a higher activation energy, which was responsible for the significant reduction in hysteresis and the improved chemical stability against a Ag electrode as compared to the corresponding characteristics of its pristine MAPbI3 counterpart. An unencapsulated MAPbI3-based device retained less than 55% of its initial PCE in a 35-day aging test, whereas a (PEA2PbI4)0.017(MAPbI3)-based device without encapsulation exhibited a promising long-term stability, retaining over 90% of its initial PCE after 42 days.]]> |
| [57] |
Lin Y, Bai Y, Fang Y J, Chen Z L, Yang S, Zheng X P, Tang S, Liu Y, Zhao J J, Huang J S . J. Phys. Chem. Lett., 2018,9:654. https://www.ncbi.nlm.nih.gov/pubmed/29350044
DOI: 10.1021/acs.jpclett.7b02679 PMID: 29350044 3 only produce (BA)2PbI4, which has better protection due to more organic ligands in (BA)2PbI4 than the mixture of 2D perovskites. Compared to BAI treatment, BA treatment results in smoother 2D perovskite layer on 3D perovskites with a better coverage. The photovoltaic devices with 2D/3D stacking structures show much improved stability in comparison to their 3D counterparts when subjected to heat stress tests. Moreover, the conversion of defective surface into 2D layers also induces passivation of the 3D perovskites resulting in an enhanced efficiency.]]> |
| [58] |
Li C P, Lv X D, Cao J, Tang Y . Chin. J. Chem., 2019,37:30.
|
| [59] |
Cho Y Y, Soufiani A M, Yun J S, Kim J C, Lee D S, Seidel J, Deng X F, Green M A, Huang S J, Ho-Baillie A W Y . Adv. Energy Mater., 2018,8:1703392.
|
| [60] |
Luo W, Wu C C, Wang D, Zhang Y Q, Zhang Z H, Qi X, Zhu N, Guo X, Qu B, Xiao L X, Chen Z J. ACS Appl. Mater. Interfaces, 2019. 11:9149. https://www.ncbi.nlm.nih.gov/pubmed/30715841
DOI: 10.1021/acsami.8b22040 PMID: 30715841 2 on the formamidinium lead iodide (FAPbI3) perovskite surface. The ODA2+ ion seems to lie horizontally on the surface of a three-dimensional perovskite due to its substitution for two FA+ ions, which could protect the bulk perovskite more effectively. The unencapsulated perovskite solar cells showed notably improved stability, which remained 92% of its initial efficiency after storing in an ambient environment for 120 days. In addition, a higher open-circuit voltage of 1.13 V compared to that of the control device (1.04 V) was obtained due to the interface energy level modification and defect passivation. A champion power conversion efficiency of 21.18% was therefore obtained with a stabilized power output of 20.64% at the maximum power point for planar perovskite solar cells.]]> |
| [61] |
Zhou Q, Liang L S, Hu J J, Cao B B, Yang L K, Wu T J, Li X, Zhang B, Gao P. Adv. Energy Mater., 2019,9:1802595.
|
| [62] |
Zhang H, Nazeeruddin M K, Choy W C H . Adv. Mater., 2019,31:1805702.
|
| [63] |
Yang S, Wang Y, Liu P, Cheng Y B, Zhao H J, Yang H G . Nat. Energy, 2016,1:15016.
|
| [64] |
Niu T Q, Lu J, Munir R, Li J B, Barrit D, Zhang X, Hu H L, Yang Z, Amassian A, Zhao K, Liu S Z. Adv. Mater., 2018,30:1706576.
|
| [65] |
Wang F, Geng W, Zhou Y, Fang H H, Tong C J, Loi M A, Liu L M, Zhao N. Adv. Mater., 2016,28:9986. https://www.ncbi.nlm.nih.gov/pubmed/27677653
DOI: 10.1002/adma.201603062 PMID: 27677653 3 films exhibit no degradation after >2800 h air exposure.]]> |
| [66] |
Chaudhary B, Kulkarni A, Jena A K, Ikegami M, Udagawa Y, Kunugita H, Ema K, Miyasaka T . ChemSusChem, 2017,10:2473. https://www.ncbi.nlm.nih.gov/pubmed/28371487
DOI: 10.1002/cssc.201700271 PMID: 28371487 3 NH3 PbI3 perovskite film affect the solar cell performance significantly and moisture sensitivity of photoactive perovskite material limits its practical applications. Herein, we show the surface modification of a perovskite film with a solution-processable hydrophobic polymer (poly(4-vinylpyridine), PVP), which passivates the undercoordinated lead (Pb) atoms (on the surface of perovskite) by its pyridine Lewis base side chains and thereby eliminates surface-trap states and non-radiative recombination. Moreover, it acts as an electron barrier between the perovskite and hole-transport layer (HTL) to reduce interfacial charge recombination, which led to improvement in open-circuit voltage (Voc ) by 120 to 160 mV whereas the standard cell fabricated in same conditions showed Voc as low as 0.9 V owing to dominating interfacial recombination processes. Consequently, the power conversion efficiency (PCE) increased by 3 to 5 % in the polymer-modified devices (PCE=15 %) with Voc more than 1.05 V and hysteresis-less J-V curves. Advantageously, hydrophobicity of the polymer chain was found to protect the perovskite surface from moisture and improved stability of the non-encapsulated cells, which retained their device performance up to 30 days of exposure to open atmosphere (50 % humidity).]]> |
| [67] |
Kim M, Motti S G, Sorrentino R, Petrozza A. Energy Envir. Sci., 2018,11:2609.
|
| [68] |
Abdelmageed G, Sully H R, Naghadeh S B Ali A E H, Carter S A, Zhang J Z . ACS Appl. Energy Mater., 2018,1:387. https://pubs.acs.org/doi/10.1021/acsaem.7b00069
|
| [69] |
Zhang C C, Li M, Wang Z K, Jiang Y R, Liu H R, Yang Y G, Gao X Y, Ma H . Mater. Chem. A, 2017,5:2572. http://xlink.rsc.org/?DOI=C6TA08970D
DOI: 10.1039/C6TA08970D |
| [70] |
Wu W Q, Yang Z B, Rudd P N, Shao Y C, Dai X Z, Wei H T, Zhao J J, Fang Y J, Wang Q, Liu Y, Deng Y H, Xiao X, Feng Y X, Huang J S. Sci. Adv., 2019. 5:eaav8925. https://www.ncbi.nlm.nih.gov/pubmed/30873433
DOI: 10.1126/sciadv.aav8925 PMID: 30873433 2) and 20.0% (aperture, 1.1 cm2), with a record-small open-circuit voltage deficit of 0.35 V under AM1.5G illumination. The stabilized PCE reaches 22.6% under 0.3 sun. Anchoring monolayer bilateral amino groups passivates the defects at the perovskite surface and enhances perovskite stability by exposing the linking hydrophobic alkyl chain. Grain boundaries are reinforced by BAA and are more resistant to mechanical bending and electron beam damage. BAA improves the device shelf lifetime to >1000 hours and operation stability to >500 hours under light, with 90% of the initial efficiency retained.]]> |
| [71] |
Lin C T, De Rossi F, Kim J, Baker J, Ngiam J, Xu B, Pont S, Aristidou N, Haque S A, Watson T, McLachlan M A, Durrant J R. J. Mater. Chem. A, 2019,7:3006.
|
| [72] |
Qin P L, Yang G, Ren Z W, Cheung S H, So S K, Chen L, Hao J H, Hou J H, Li G. Adv. Mater., 2018,30:1706126.
|
| [73] |
Li X, Chen C C, Cai M L, Hua X, Xie F X, Liu X, Hua J L, Long Y T, Tian H, Han L Y. Adv. Energy Mater., 2018,8:1800715.
|
| [74] |
Zhang W W, Lei X L, Liu J H, Dong J, Yan X W, Gao W, Dong H, Ran C X, Wu Z X . Phys. Status Solidi (RRL), 2019,13:1800505.
|
| [75] |
Zhao S H, Xie J S, Cheng G H, Xiang Y R, Zhu H Y, Guo W Y, Wang H, Qin M C, Lu X H, Qu J L, Wang J N, Xu J B, Yan K Y . Small, 2018,14:1803350.
|
| [76] |
Dequilettes D W, Koch S, Burke S, Paranji R K, Shropshire A J, Ziffer M E, Ginger D S. ACS Energy Lett., 2016,1:438. https://pubs.acs.org/doi/10.1021/acsenergylett.6b00236
|
| [77] |
Zhang H, Wu Y Z, Shen C, Li E P, Yan C X, Zhang W W, Tian H, Han L Y, Zhu W H. Adv. Energy Mater., 2019: 1803573.
|
| [78] |
You S, Wang H, Bi S Q, Zhou J Y, Qin L, Qiu X H, Zhao Z Q, Xu Y, Zhang Y, Shi X H, Zhou H Q, Tang Z Y. Adv. Mater., 2018,30:1706924.
|
| [79] |
Ono L K, Qi Y B . J. Phys. Chem. Lett., 2016,7:4764. https://www.ncbi.nlm.nih.gov/pubmed/27791377
DOI: 10.1021/acs.jpclett.6b01951 PMID: 27791377 The current challenges (e.g., stability, hysteresis, etc.) in organometal halide perovskite solar cell research are closely correlated with surfaces and interfaces. For instance, efficient generation of charges, extraction, and transport with minimum recombination through interlayer interfaces is crucial to attain high-efficiency solar cell devices. Furthermore, intralayer interfaces may be present in the form of grain boundaries within a film composed of the same material, for example, a polycrystalline perovskite layer. The adjacent grains may assume different crystal orientations and/or have different chemical compositions, which impacts charge excitation and dynamics and thereby the overall solar cell performance. In this Perspective, we present case studies to demonstrate (1) how surfaces and interfaces can impact material properties and device performance and (2) how these issues can be investigated by surface science techniques, such as scanning probe microscopy, photoelectron spectroscopy, and so forth. We end this Perspective by outlining the future research directions based on the reported results as well as the new trends in the field. |
| [80] |
She L M, Liu M Z, Zhong D Y . ACS Nano, 2015,10:1126. https://www.ncbi.nlm.nih.gov/pubmed/26643387
DOI: 10.1021/acsnano.5b06420 PMID: 26643387 We report on the atomic structures of methylammonium (MA) lead iodide (CH3NH3PbI3) perovskite surfaces, based on a combined scanning tunneling microscopy and density functional theory calculation study. A reconstructed surface phase with iodine dimers, coexisting with the pristine zigzag phase, was found at the MA-iodine-terminated (001) surfaces of the orthorhombic perovskite films grown on Au(111) surfaces. The reorientation of surface MA dipoles, which strengthens the interactions with surface iodine anions, resulting in a slight energy reduction of 34 meV per unit cell, is responsible for the surface iodine dimerization. According to our calculation, the surface MA dipoles weaken the surface polarity and are therefore considered to be stabilizing the surface structures. |
| [81] |
Ohmann R, Ono L K, Kim H S, Lin H P, Lee M V, Li Y Y, Park N G, Qi Y B . J. Am. Chem. Soc., 2015,137:16049. https://www.ncbi.nlm.nih.gov/pubmed/26639900
DOI: 10.1021/jacs.5b08227 PMID: 26639900 Organic-inorganic perovskite is a promising class of materials for photovoltaic applications and light emitting diodes. However, so far commercialization is still impeded by several drawbacks. Atomic-scale effects have been suggested to be possible causes, but an unequivocal experimental view at the atomic level is missing. Here, we present a low-temperature scanning tunneling microscopy study of single crystal methylammonium lead bromide CH3NH3PbBr3. Topographic images of the in situ cleaved perovskite surface reveal the real-space atomic structure. Compared to the bulk we observe modified arrangements of atoms and molecules on the surface. With the support of density functional theory we explain these by surface reconstruction and a substantial interplay of the orientation of the polar organic cations (CH3NH3)(+) with the position of the hosting anions. This leads to structurally and electronically distinct domains with ferroelectric and antiferroelectric character. We further demonstrate local probing of defects, which may also impact device performance. |
| [82] |
Baumann A, Vath S, Rieder P, Heiber M C, Tvingstedt K, Dyakonov V . J. Phys. Chem. Lett., 2015,6, 2350. https://www.ncbi.nlm.nih.gov/pubmed/26266616
DOI: 10.1021/acs.jpclett.5b00953 PMID: 26266616 Thermally stimulated current (TSC) measurements are used to characterize electronic trap states in methylammonium lead iodide perovsite solar cells. Several TSC peaks were observed over the temperature range from 20 K to room temperature. To elucidate the origins of these peaks, devices with various organic charge transport layers and devices without transport layers were tested. Two peaks appear at very low temperatures, indicating shallow trap states that are mainly attributed to the PCBM/C60 electron transport bilayer. However, two additional peaks appear at higher temperatures, that is, they are deeper in energy, and are assigned to the perovskite layer. At around T = 163 K, a sharp peak, also present in the dark TSC measurements, is assigned to the orthorhombic-tetragonal phase transition in the perovskite. However, a peak at around T = 191 K is assigned to trap states with activation energies of around 500 meV but with a rather low concentration of 1 × 10(21) m(-3). |
| [83] |
Heo S, Seo G, Lee Y, Lee D, Seol M, Lee J, Park J B, Kim K, Yun D J, Kim Y S, Shin J K, Ahn T K, Nazeeruddin M K. Energy Environ. Sci., 2017,10:1128.
|
| [84] |
Chen B, Rudd P N, Yang S, Yuan Y B, Huang J S . Chem. Soc. Rev., 2019,48:3842. https://www.ncbi.nlm.nih.gov/pubmed/31187791
DOI: 10.1039/c8cs00853a PMID: 31187791 All highly-efficient organic-inorganic halide perovskite (OIHP) solar cells to date are made of polycrystalline perovskite films which contain a high density of defects, including point and extended imperfections. The imperfections in OIHP materials play an important role in the process of charge recombination and ion migration in perovskite solar cells (PSC), which heavily influences the resulting device energy conversion efficiency and stability. Here we review the recent advances in passivation of imperfections and suppressing ion migration to achieve improved efficiency and highly stable perovskite solar cells. Due to the ionic nature of OIHP materials, the defects in the photoactive films are inevitably electrically charged. The deep level traps induced by particular charged defects in OIHP films are major non-radiative recombination centers; passivation by coordinate bonding, ionic bonding, or chemical conversion have proven effective in mitigating the negative impacts of these deep traps. Shallow level charge traps themselves may contribute little to non-radiative recombination, but the migration of charged shallow level traps in OIHP films results in unfavorable band bending, interfacial reactions, and phase segregation, influencing the carrier extraction efficiency. Finally, the impact of defects and ion migration on the stability of perovskite solar cells is described. |
/
| 〈 |
|
〉 |