1 引言
2 癌症与自噬
2.1 自噬抑制癌症的发生
2.2 自噬促进癌症的发展
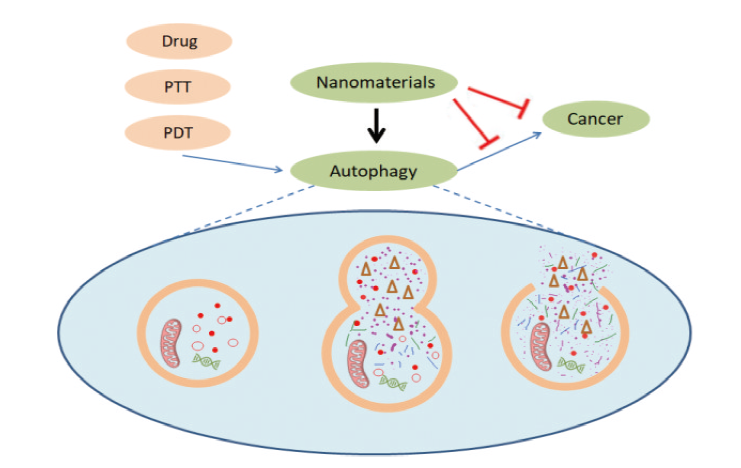
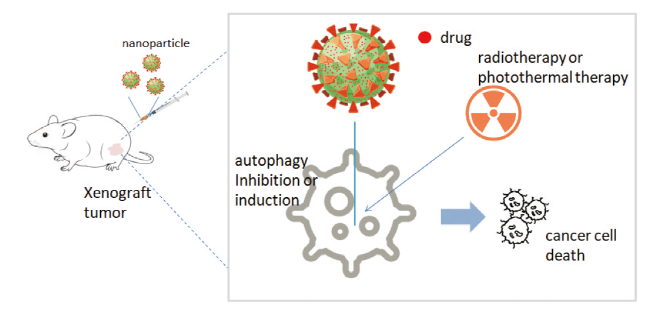
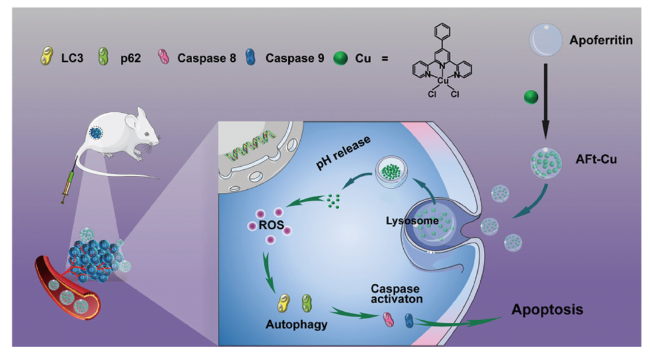
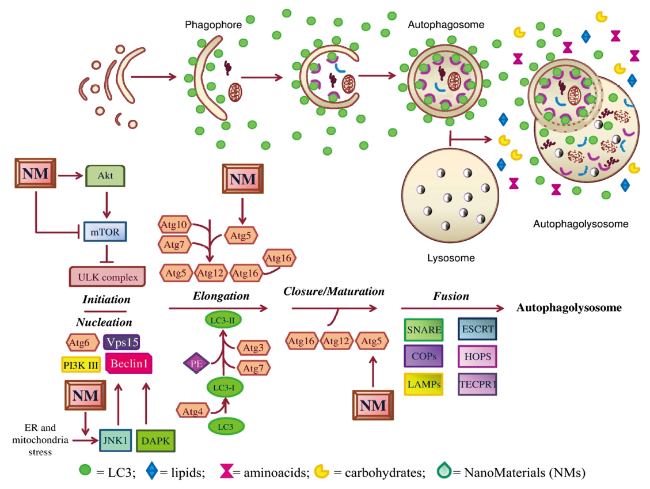
3 纳米材料对自噬的影响
表1 纳米材料通过调控自噬治疗癌症Table 1 Nanomaterials treat cancer by regulating autophagy |
| NMs | Size (nm) | shape or dispersity | Coating | Drug or therapies | Model cells | Mechanism | Autophagy | Effect | ref |
|---|---|---|---|---|---|---|---|---|---|
| Au NPs | 20 | sphere | PEG | immunotherapy | Hepa1-6 cells; RAW 264.7 cells | Lysosome alkalization; membrane permeabilization | Inhibition | Increased sensitivity | 79 |
| Au NPs | 30~60 | Peanut | Bare | — | SKOV-3 cell | ROS upregulation | Inhibition | Apoptosis | 78 |
| Ag NPs | 66.92 | sphere | Bare | — | PC-3 cell | lysosome injury; cell hypoxia | Inhibition | Cell death | 93 |
| Fe3O4-Au NPs | 15~25 | sphere | — | DOX | HepG2 cells | Enhancing autophagosome formation | Induction | Reduce drug resistance | 80 |
| Ag NPs | 59 | sphere | Bare | — | HT-29 cells | JNK activation and eIF2α phosphorylation | Induction | Apoptosis | 97 |
| Fe@Au NPs | — | core-shell structure | — | — | OECM1 cell | Mitochondria damage | Inhibition | Cell death | 73 |
| Ag NPs | 26.5 | sphere | PVP | — | HeLa cell | PtdIns3K-dependent | Induction | Ehances the anticancer activity | 90 |
| Fe3O4 NPs | 36 | sphere | PEG | PTX | U251 cell | ROS upregulation | Induction | Reduce drug resistance | 98 |
| Ag NPs | 13 | sphere | — | — | A549 cell | ROS upregulation | Induction | Apoptosis | 83 |
| Ag NPs | 8 | sphere | protein | Cisplatin | OS cell; HCC cell | MAPK pathways | Induction | Reduce drug resistance | 89 |
| Fe2O3 NPs | 10 | sphere | DMSA | — | SK-Hep-1 cell | ROS upregulation; MAPK pathways | Induction | Cell death | 99 |
| CuO NPs | 10 | sphere | — | — | MCF7 cell | ROS dependent | Induction | Growth inhibition | 100 |
| ZnO NPs | 21 | — | — | Sorafenib | Huh 7 cell | Promoting p53 Gene | Induction | Apoptosis | 101 |
| ZnO NPs | 63 | — | — | — | MCF7 cell | — | Inhibition | Apoptosis | 102 |
| SiO2 NPs | 86 | sphere | HCT-116 cells | ER stress | Induction | Cell survival | 103 | ||
| Ag NPs | 15.38 | sphere | PVP | Radiotherapy | U251 cells | ROS upregulation | Induction | Increased sensitivity | 88 |
| IONPs | 37 | — | PEG | — | U251 cells | Beclin 1/ATG 5 pathways | Induction | Ferroptosis | 30 |
| Gd2O3 NPs | — | — | — | Cisplatin | HeLa cells | — | Inhibition | Reduce drug resistance | 104 |
| SiO2 NPs | 125 | sphere | — | Propranolol | HemSCs cells | ER stress | Induction | Cell death | 105 |
| ZnO NPs | 20 | — | — | — | SKOV3 cells | ROS upregulation | Induction | Apoptosis | 106 |
| SiO2 NPs | 198 | sphere | PDA; PEG | DOX | MCF7 cells | AKT-mTOR-p70S6K pathway | Induction | Cell death | 40 |
| ND | 191 | — | — | Hypoxia | HeLa cells; MCF7 cells | — | Inhibition | Apoptosis | 107 |
| ZnO NPs | — | — | — | Cisplatin | SGC7901 cells;BGC823 cells | — | Inhibition | Reduce drug resistance | 108 |
| GO | 450 | sheet | DMSO | Cisplatin | Skov-3 cells | — | Induction | Cell death | 109 |
| DWCNTs | — | Tube | — | — | DHD/K12/Trb cell line | Intracellular acidification | Induction | Cell death | 110 |
| Se NPs | 70 | Amorphous solid | — | Astragalus Polysaccharides | MCF7 cells | ROS upregulation and Mitochondria damage | Inhibition | Apoptosis | 111 |
| BPQDs | 140 | Monodisperse | Platelet membrane | Hederagenin | MCF7 cells; RAW 264.7 cells | ROS upregulation and Mitochondria damage | Induction | Apoptosis | 112 |
| Co3O4 NPs | 200 | sphere | — | Photothermal therapy | U-87 MG cells | Llysosomal function damage | Blockage of autophagic flux | Cell death | 113 |