1 引言
1.1 太阳能界面水汽转换简介
1.2 ISSG中材料体系的设计思路和实例分析
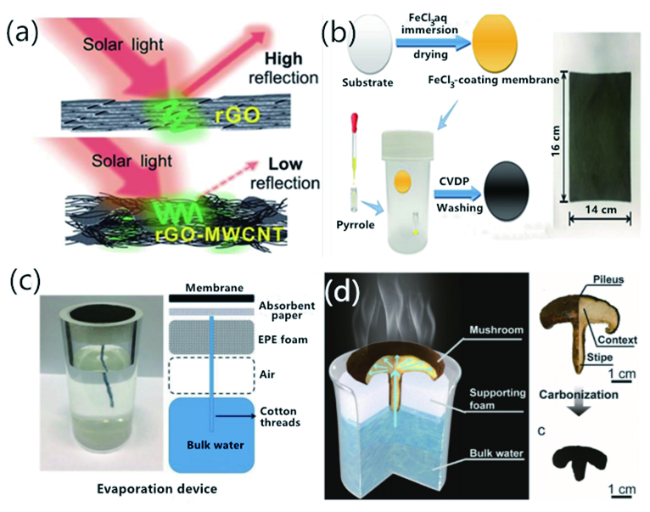
图1 (a)rGO-MWCNTs复合薄膜[59];CVPD制备PPy薄膜(b)[60]、三明治(c)[60]和蘑菇结构[62](d)的太阳能界面水汽转换材料体系Fig. 1 (a) rGO-MWCNTs membrane[59]. Copyright 2018, Royal Society of Chemistry;(b) PPy membrane made from CVPD[60]. Solar steam generation system of sandwich(c)[60] and mushroom structure(d)[62]. Copyright 2018, John Wiley and Sons. Copyright 2017, John Wiley and Sons |
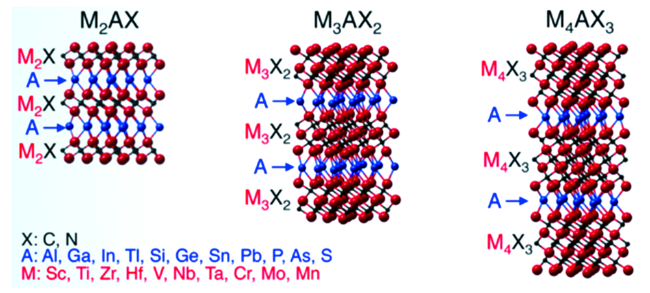
1.3 Ti3C2-MXene的简介
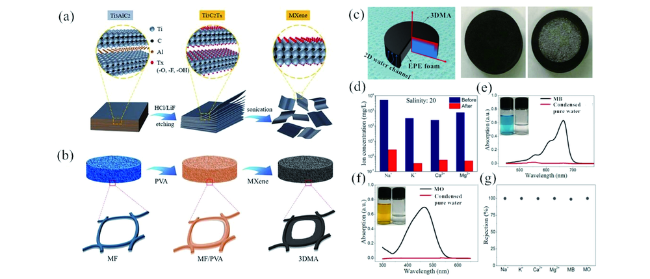
图3 SEM图像:刻蚀前的Ti3AlC2(a,c,e,i,k);HF刻蚀制备的Ti3C2-MXene(b)[96];HCl/LiF刻蚀制备的Ti3C2-MXene(d)[97];NaHF2(f)、KHF2(g)和NH4HF2(h)刻蚀制备的Ti3C2-MXene[98];浓碱法刻蚀制备的Ti3C2-MXene(j)[99];NaOH和H2SO4刻蚀制备的Ti3C2-MXene(l)[100];HR-TEM图像:电化学刻蚀制备前(m)后(n)的Ti3AlC2[101]Fig. 3 SEM images of Ti3AlC2 before etching(a, c, e, i, k); HF etching prepared Ti3C2-MXene[96](b). Copyright 2012, American Chemical Society; HCl/LiF etching prepared Ti3C2-MXene[97](d). Copyright 2019, John Wiley and Sons; NaHF2(f), KHF2(g) and NH4HF2(h) etching prepared Ti3C2-MXene[98]. Copyright 2017, Elsevier; Concentrated alkali etching prepared Ti3C2-MXene[99](j). Copyright 2018, WILEY-VCH; NaOH/H2SO4 etching prepared Ti3C2-MXene[100](l). Copyright 2014, Royal Society of Chemistry; HR-TEM images of Ti3AlC2 before(m) and after(n) electrochemical etching[101]. Copyright 2018, WILEY-VCH |
1.4 Ti3C2-MXene在太阳能界面水汽转换的应用
2 Ti3C2在太阳能界面水汽转换的研究进展
表1 Ti3C2-MXene复合材料在太阳能水汽转换的部分参数Table 1 Summary of Ti3C2-MXene for solar steam generation |
| Solar intensity (1 Sun=1 kW·m-2) | Material | Efficiency(%) | Evaporation ratea (kg·m-2·h-1) | ref |
|---|---|---|---|---|
| 1 Sun | Ti3C2-MXene/Cellulose membrane | 85.8 | 1.44 | 112 |
| 1 Sun | Ti3C2-MXene/PVDF membrane | 84 | - | 107 |
| 1 Sun | Hydrophobic d-Ti3C2 membrane | 71 | 1.31 | 54 |
| 1 Sun | PDA@MXene PVDF membrane | 85.2 | 1.276 | 113 |
| 1 Sun | Cellulose acetate-MXene membrane(CAM) | 92.1 | 1.47 | 114 |
| 1 Sun | Tree-inspired hydrogel(TIH) | 90.7 | 2.71 | 49 |
| 1 Sun | Janus Vertically aligned MXene aerogel (Janus VA-MXA) | 87 | 1.46 | 53 |
| 1 Sun/0.5 Sun and 2.5 V voltage | Crosslinked MXene Aerogels(CMA) | - | 1.337/1.624 | 128 |
| 1 Sun | GO/Ti3C2-MXene Aerogel(GMA) | 90.7 | 1.27 | 129 |
| 1 Sun | 3D CMF@d-Ti3C2 | 84.6 | 1.60 | 51 |
| 1 Sun | Three-dimensional MXene architectures(3DMAs) | 88.7 | 1.41 | 130 |
| 1 Sun | Ti3C2-Wood | 96 | 1.465 | 135 |
aAt the same evaporation rate, some of the differences in the efficiency of solar steam generation are caused by the fact that some articles do not deduct the rate of natural evaporation of water or do not include sensible heat in the calculation |
2.1 二维MXene薄膜
2.1.1 浸涂烘干法
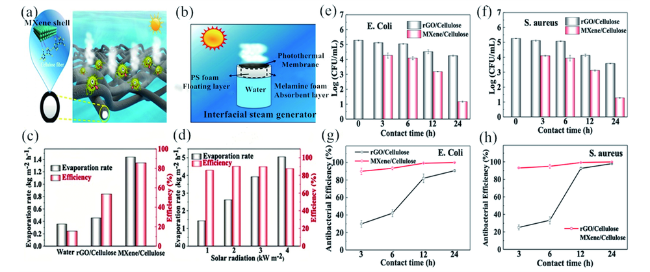
图4 (a)抗菌Ti3C2-MXene/纤维素薄膜;(b)由PS和CMF组成的材料体系;(c)1 Sun下纯水、rGO/纤维素薄膜和Ti3C2-MXene/纤维素薄膜的水蒸发速率和光热水汽转换效率;(d)不同光照强度下Ti3C2-MXene/纤维素薄膜的水蒸发速率和光热水汽转换效率;rGO/纤维素薄膜和Ti3C2-MXene/纤维素薄膜对大肠杆菌(e,g)和金黄色葡萄球菌(f,h)的抗菌效果[112]Fig. 4 (a) Antibacterial Ti3C2-MXene/ cellulose membrane;(b) system composed of PS and CMF;(c) water evaporation rates and solar steam efficiency of bulk water, rGO/cellulose, and Ti3C2-MXene/cellulose membranes under the solar illumination of 1 sun;(d) water evaporation rates of Ti3C2-MXene/cellulose membrane under the solar illumination of different intensities; antibacterial performance of rGO/cellulose and Ti3C2-MXene/cellulose membranes for E. coli (e, g) and S. aureus(f, h)[112]. Copyright 2019, American Chemical Sociey |
2.1.2 过滤法
图5 (a)疏水d-Ti3C2膜的制备过程和(b)材料体系结构;亲、疏水d-Ti3C2膜经过24 h海水淡化前(c,e)和后(d,f)的照片;亲水(g)和疏水d-Ti3C2膜(h)太阳能海水淡化过程;(i)海水淡化前后四种主要离子浓度的变化;(j)有机溶液和重金属离子的净化效率[54]Fig. 5 (a) Fabrication process and(b) system architecture of the hydrophobic d-Ti3C2 membrane; optical photographs of the hydrophilic and hydrophobic d-Ti3C2 membranes before(c, e) and after(d, f) 24 h solar desalination; solar desalination process of(g) hydrophilic and(h) hydrophobic d-Ti3C2 membranes;(i) measured salinity of four primary ions before and after solar desalination;(j) organic and heavy metal ion rejection performance[54]. Copyright 2018, Royal Society of Chemistry |
图6 (a)制作PDA@MXene光吸收层和(b)材料体系结构的示意图;(c)1 Sun下不同样品的水量变化;(d)不同样品在不同光强照射下的水蒸发速率和(e)光热水汽转换效率[113]Fig. 6 Schematic illustration of the(a) fabrication of PDA@MXene light absorption layer and(b) system architecture;(c) mass change of water with different samples under the solar illumination of 1 sun;(d) water evaporation rates and(e) solar steam efficiency of different samples under different solar illumination intensities[113]. Copyright 2019, Springer Nature |
2.1.3 其他
2.2 三维MXene复合材料
2.2.1 水凝胶
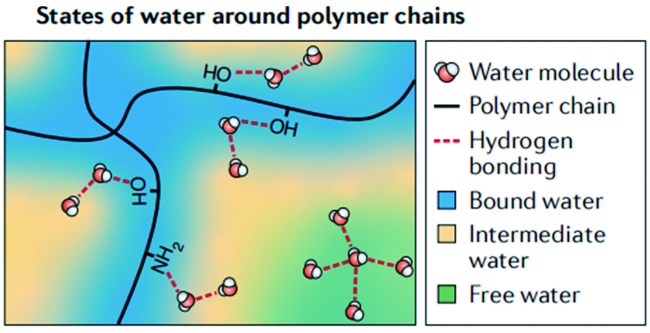
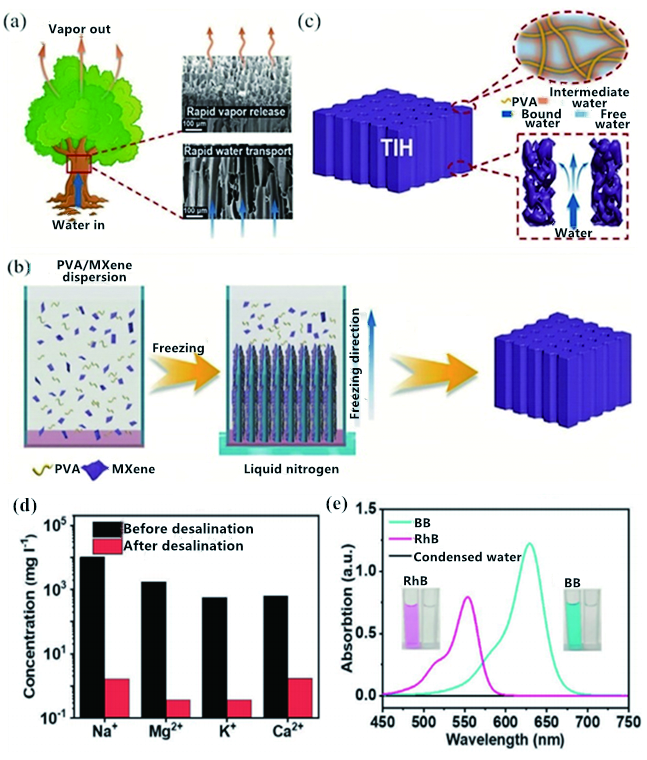
图9 (a)树木将水从根部输送到树干顶部的示意图,树中垂直排列的微通道提供的开放通道有利于水的运输和蒸汽的逸出。右边为木材顶部和横截面(底部)的SEM图;(b)TIH的制备过程示意图;(c)TIH的原理图:水通过垂直排列的通道运输(右下)以及水分子与分子网格(右上)的相互作用调节水分蒸发焓和水分蒸发焓;(d)海水淡化前后四种主要离子的浓度;(e)水净化前后BB和RhB水溶液的紫外-可见光谱[49]Fig. 9 (a) Schematic diagram of water being transferred from root to top of trunk by tree, open channels provided by vertically aligned microchannels in tree are beneficial to water transport and vapor release. The scanning electron microscopy(SEM) images on the right are the top view(top) and cross-section(bottom) of wood;(b) schematic illustration of the preparation process of TIH;(c) schematic of the TIH, water transport through vertically aligned channels(lower right) and the water evaporation enthalpy can be tuned by the interaction between water molecules and molecular mesh(upper right);(d) the concentration of four main ions in seawater before and after desalination;(e) UV-vis spectroscopy of BB and RhB aqueous solutions before and after water purification[49]. Copyright 2020, WILEY-VCH |
2.2.2 气凝胶
图10 (a)Janus VA-MXA的制备过程;Janus VA-MXA的(b)俯视、(c)侧视和(d)断面图像;(e)Janus VA-MXA上层的SEM图像[53]Fig. 10 (a) Fabrication process of a Janus VA-MXA; digital photograph of the(b) top view,(c) side view and(d) the fracture face of the as-prepared Janus VA-MXA;(e)SEM image of the upper layer of prepared Janus VA-MXA[53]. Copyright 2020, American Society Chemistry |
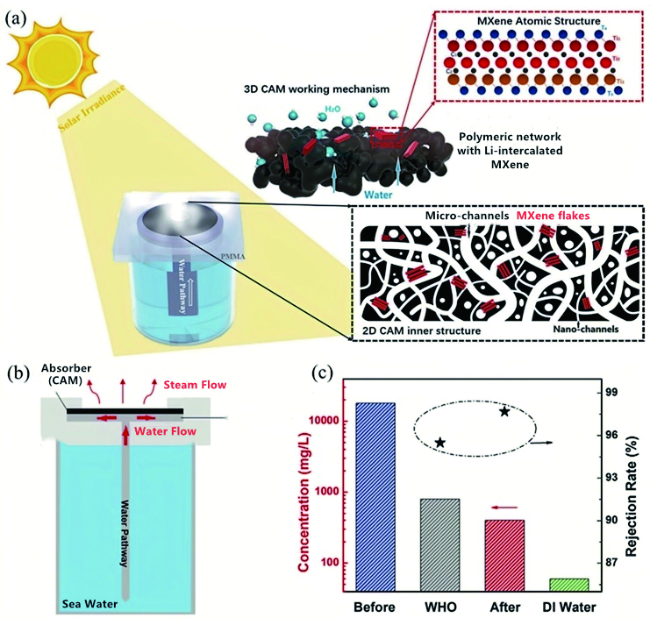
图11 (a)CMA基于协同光热和电热转换的全天候蒸气生成系统的概念图;(b)CMA的制造工艺示意图;(c)标准海水样品中四种主要离子在蒸发前后的浓度测量;(d)在0.5 Sun和5 V电压供电的组合下,CMA的水分蒸发稳定性超过20个周期,内嵌:水在第一周期和第20周期的质量变化;(e)太阳能电池将阳光转化为电力并将其存储到电池中,以便进一步为CMA供电的示意图;(f)一套大规模蒸气发电系统的光学照片;(g)上午9:00至下午23:00,SC-B组件的充电电流密度;(h)有无SC-B组件,CMA的水蒸发速率[128]Fig. 11 (a) Conceptual schematic of the all-weather steam generation system based on the synergistic photo-thermal and electro-thermal conversion of CMA;(b) schematic illustration for the fabrication process of CMA;(c) the measured concentrations of four primary ions in a standard seawater sample before(original) and after evaporation desalination;(d) the water evaporation durability performance of CMA under the combined solar illumination of 0.5 sun and voltage supply of 5 V over 20 cycles, and insets of(d) are the mass change of water with the CMA in the 1st cycle and 20th cycle;(e) schematic illustration showing that the solar cells convert sunlight into electricity and store it into battery for further powering the steam generator;(f) optical photographs of a set of large-scale steam generation system;(g) the charging current density of SC-B components at different times from 9:00 am to 23:00 pm;(h) the water evaporation rates at different times in two situations, that is with SC-B or without SC-B[128]. Copyright 2019, Royal Society of Chemistry) |
图13 以GMA-3为基础进行的工作:(a)太阳能驱动界面海水淡化装置示意图;(b)太阳能驱动界面海水淡化前后人工海水中5种离子浓度变化;(c)1 Sun光强照射下,在纯水和海水中进行30个周期稳定性试验,每个周期30 min;(d)太阳能驱动的水净化前后,人工废水中7种主要金属离子的浓度变化;太阳能驱动的水净化前后,GMA对自然湖水(武汉沙湖)和人工细菌溶液(大肠杆菌)的(e)灭菌结果和(f)数据,(e)内嵌图为大肠杆菌溶液的照片;太阳能驱动的水净化(g,h)前后,油/水混合乳液的光学显微镜图像,(h)内嵌图为油/水混合乳液在净化前(左)和净化后(右)的照片;(i)对油/水混合乳液进行太阳能驱动的水净化稳定性试验;(j)油/水混合乳液净化后的水纯度[129]Fig. 13 GMA-3 based work(a) schematic illustration of a designed solar-driven interfacial desalination still;(b) concentrations of five primary ions in the artificial seawater before and after solar driven desalination;(c) endurance tests in pure water and seawater for 30 cycles under 1 sun, Each cycle is 30 mins;(d) concentrations of seven primary metal ions in artificial wastewater before and after solar-driven purification;(e) sterilization results and(f) performance of natural lake water(Sha Lake, Wuhan) and artificial bacterial solution(E. coli) before and after solar-driven interfacial evaporation, respectively. Inset of(e), the photograph of E. coli solution;(g, h) optical microscopy images of emulsified oil/water mixtures before and after solar-driven separation, Inset of(h), the photograph of emulsified oil/water mixtures before(left) and after(right) separation;(i) the cyclic test of solar-driven water purification for emulsified oil/water mixtures;(j) the water purity of emulsified oil/water mixtures after purification[129]. Copyright 2020, Elsevier |
2.2.3 其他
图14 (a)3D CMF@d-Ti3C2的制备工艺图;(b)基于3D CMF@d-Ti3C2的太阳能界面转换材料体系;1 Sun下3D CMF@d-Ti3C2和二维d-Ti3C2膜的(c)水量变化与对应的(d)光热水汽转换效率[51]Fig. 14 (a) Schematic illustration of the fabrication procedures of the 3D CMF@d-Ti3C2;(b) the 3D CMF@d-Ti3C2 based solar evaporator;(c) mass change of water through evaporation and the corresponding(d) solar to vapor conversion efficiency of the 3D CMF@d-Ti3C2 and the 2D d-Ti3C2 membrane[51]. Copyright 2019, World Scientific Publishing Co. Pte Ltd |
图15 (a)HF刻蚀法制备Ti3C2-MXene的工艺图;(b)3DMAs的制备工艺图;(c)嵌入EPE的材料体系结构;(d)盐度为20的标准海水样品中的4种主要离子在水净化前后的测量浓度变化;甲基蓝(MB)和甲基橙(MO)溶液在水净化前后(e,f)的吸收光谱图,(e,f)内嵌图为MB和MO水净化前后照片;(g)太阳能界面海水淡化和废水净化后,金属离子和有机染料的净化率[130]Fig. 15 (a) HF etching process diagram for preparation of Ti3C2-MXene;(b) preparation process diagram of 3DMAs;(c) embedded EPE architecture;(d) the measured concentrations of four primary ions in a standard seawater sample with salinity of 20 before(original) and after evaporation; absorption spectra of methylene blue(MB) and methyl orange(MO) solutions before evaporation(black line) and corresponding condensed pure water after evaporation(red line), respectively(e, f), inset in(e, f) is the optical photographs of MB and MO before and after evaporation;(g) metal ion and organic dye rejection performance undergoing solar seawater desalination and wastewater purification[130]. Copyright 2019, Royal Society of Chemistry |
2.3 生物基或仿生MXene复合材料
2.3.1 圆木片生物基复合材料
2.3.2 仿生复合材料
图17 (a)西非加蓬蝰蛇的照片;(b)黑色背部鳞片的照片(左)和SEM图像(右),黑色鳞片表面覆盖着密集的微冠结构;(c)黑色鳞片的高倍SEM图像,微冠结构上有支化的纳米结构;(d)G1 MXene纳米涂层的照片(左,比例尺:1 cm)和SEM图像(右,比例尺:10 μm);(e)分层MXene纳米涂层的SEM图像(比例尺:30 μm);(f)具有宽带光吸收和增强光热性能的仿生MXene纳米涂层示意图;(g)用于高效光热转换的仿生MXene纳米涂层ISSG材料装置的示意图[136]Fig. 17 (a) Digital photograph of Bitis rhinoceros, the West African Gaboon viper;(b) digital photograph(left) and SEM image(right) of the black dorsal scales of Bitis rhinoceros, the surface of the black scales was covered with intensive microcrest structures;(c) high magnitude SEM image of the black scale, branched nanoridges were present on the microcrest structures;(d) digital photograph(left, scale bar 1 cm) and SEM image(right, scale bar 10 μm) of the G1 MXene nanocoating;(e) SEM image of the hierarchical MXene nanocoating(scale bar 30 μm);(f) schematic illustration of the biomimetic MXene nanocoating with broadband light absorption and enhanced light-to-heat performance;(g) schematic illustration of the solar steam-generation device with the bioinspired MXene nanocoating for high solar-thermal conversion[136]. Copyright 2019, WILEY-VCH |
图18 (a)仿生三维球形结构的太阳能界面水汽转换材料;(b)Co3O4/Ti3C2-MXene光吸收层的制备图; (c)1 Sun光强照射下,不同光照角度的三维CM-0.1/织物球体的水分蒸发重量随时间的变化;(d)二维和三维结构的水汽转换材料的水蒸发速率及相较于空白样品相应效率增强因子;(e)蒸发前模拟海水及冷凝水中Na+的重量百分比(1.4 wt%、3.5 wt%、4.1 wt%);(f)模拟的废水净化表现:MO(464 nm)和MB(663 nm)的吸收峰在冷凝水中消失;(g)ICP测定的重金属离子污水和冷凝水中Cu2+、Pb2+、Cd2+的离子浓度;(h)在1 Sun光强照射下,对三维 CM-0.1/织物球体进行循环太阳蒸发测试[137]Fig. 18 (a) The biomimetic architectural structure of 3D spherical evaporator;(b) schematic illustration showing the stepwise preparation of Co3O4/ Ti3C2-MXene composites;(c) cumulative weight loss of 3D CM-0.1/fabric sphere through water evaporation over time under 1.0 sun illumination in different angles;(d) evaporation rate and corresponding enhancement factor of efficiency for 2D and 3D evaporator in comparison to the blank sample;(e) the weight percentage of Na+ of the condensed water evaporated from the simulated seawater(1.4 wt%, 3.5 wt%, 4.1 wt%);(f) simulated wastewater purified performance; the absorption peaks of MO(at 464 nm) and MB(at 663 nm) disappeared in the condensed water;(g) the ion concentration of Cu2+, Pb2+, and Cd2+ in heavy metal ion sewage and purified water obtained by ICP measurement;(h) the cycling solar evaporation measurements for 3D CM-0.1/fabric sphere under 1.0 sun illumination[137]. Copyright 2020, WILEY-VCH |