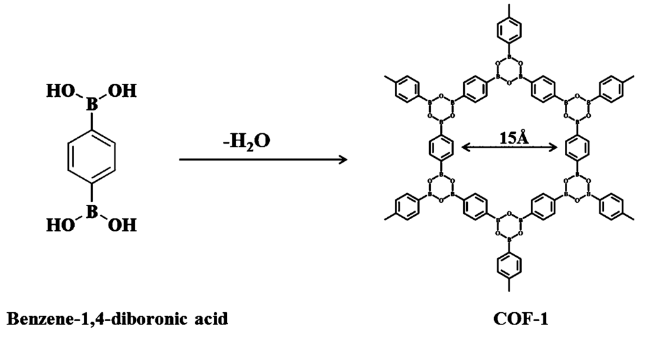
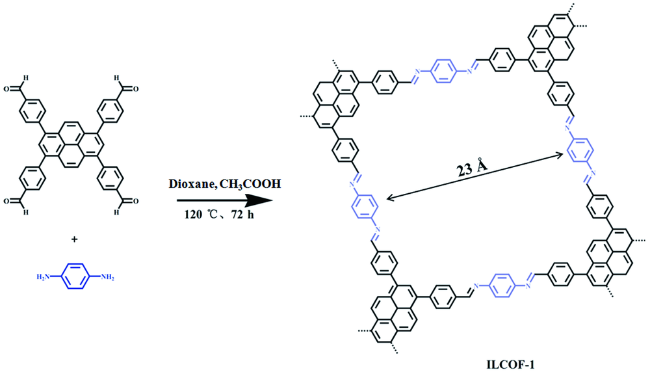
1 引言
2 共价有机框架材料的结构控制和分类
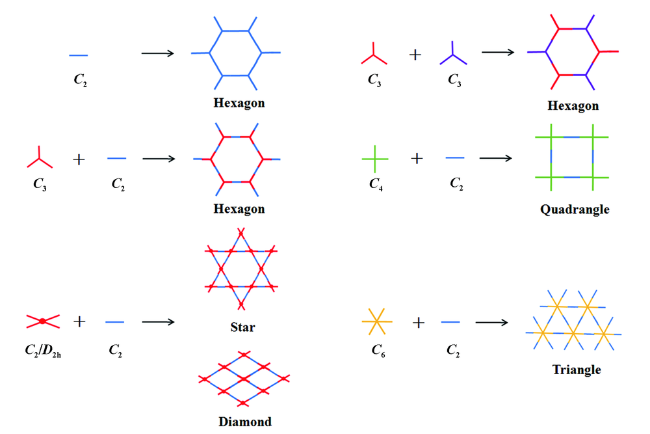
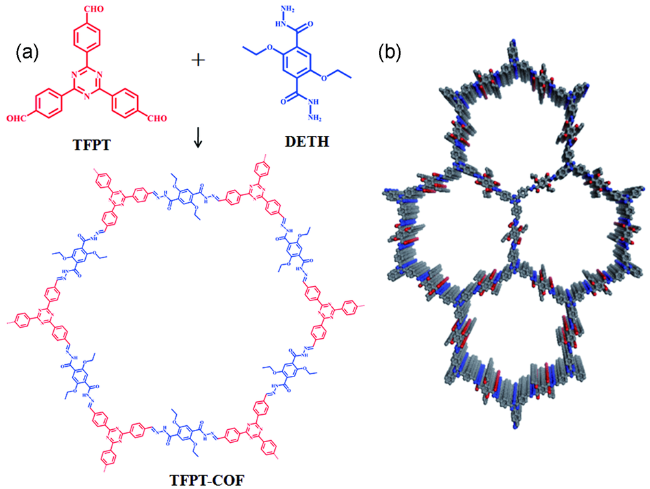
2.1 构筑单体(Units)
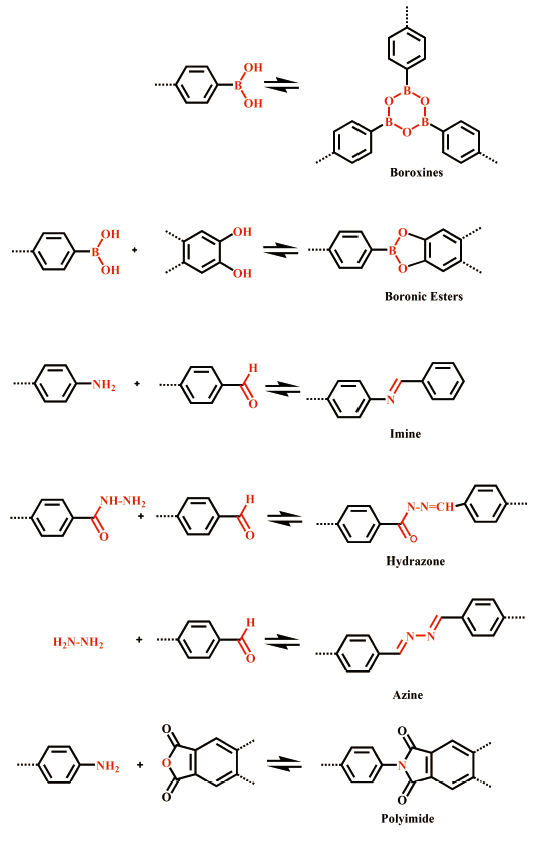
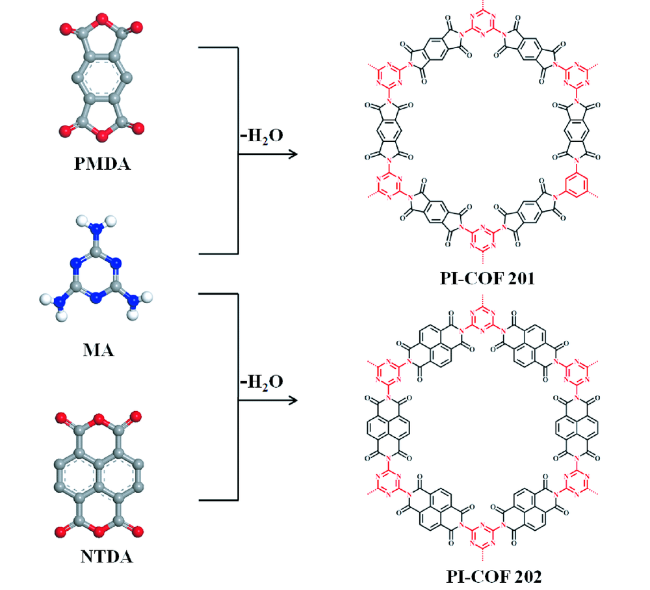
2.2 链接键(Linkages)
3 COFs材料用于去除环境污染物
3.1 离子类
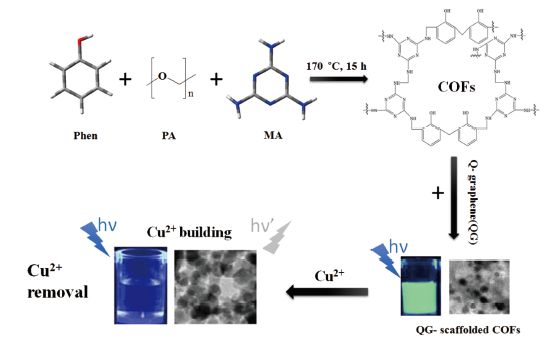
图5 石墨烯(QG)搭架COFs材料作为荧光探针和吸附剂用于铜离子的检测和去除。苯酚(Phen)、多聚甲醛(PA)和三聚氰胺(MA)共价聚合反应生成COFs材料。该COFs材料与QG搭架制备无金属QG-搭架COFs,得到可被Cu2+猝灭的亮绿色荧光产物,其可用于检测和去除Cu2+[5]Fig.5 The application of QG-COFs in detection and removal of Cu2+ ions. The COFs are synthesized through the covalent polymerization reaction among phenol(Phen), paraformaldehyde(PA), and melamine(MA). The metal-free QG-scaffolded COFs can be quenched by Cu2+ ions and used for the detection and removal of Cu2+ ions[5] |
表1 COFs材料去除离子类污染物Table 1 Applications of COFs materials in removing ionic pollutants |
| COFs | Removal | Building units | Pore size/nm | Conditions | Adsorption capacity | ref | |
|---|---|---|---|---|---|---|---|
| pH | T/K | ||||||
| TAPB-BMTTPA-COF | Hg(Ⅱ) | TAPB, DMTTPA | 3.2 | 7.0 | 298 | 734 mg/g | 60 |
| COF-LZU8 | Hg(Ⅱ) | - | 1.23 | - | - | - | 61 |
| CTF-1 | Cd(Ⅱ) | 1,4-dicyanobenzene | 1.2 | - | 298 | 29.26 mg/g | 54 |
| γ-Fe2O3@CTF-1 | As(Ⅲ) | Fe2O3, CTF-1 | - | 7.0 | 298 | 198.0 mg/g | 62 |
| As(Ⅵ) | 102.3 mg/g | ||||||
| Hg(Ⅱ) | 165.8 mg/g | ||||||
| COF-S-SH | Hg | COF-V, thiol/thioether | 2.8 | - | 298 | 863 mg/g | 63 |
| Hg(Ⅱ) | 1350 mg/g | ||||||
| COF-TE | Pb(Ⅱ) | TMC, EDA | - | - | 298 | 185.7 mg/g | 64 |
| COF-TP | Pb(Ⅱ) | TMC, PDA | - | - | 298 | 140.0 mg/g | 64 |
| TPB-BT-COF | Cr(Ⅵ) | BT, TPB | 3.26 | - | - | - | 65 |
| TAPT-BT-COF | Cr(Ⅵ) | BT, TAPT | 3.26 | - | - | - | 65 |
| QG-scaffolded COFs | Cu(Ⅱ) | PA, MA, Phen, QG | - | - | - | - | 5 |
| 3D-OH-COF | Sr(Ⅱ) | TFPM, DHBD | 1.31 | - | - | - | 66 |
| Fe(Ⅲ) | - | ||||||
| Nd(Ⅲ) | - | ||||||
| TTTAT | I2 | TTAT | 1.22 | - | 350 | 3.41 g/g | 67 |
| TTDAT | I2 | TTDT | 1.22 | - | 350 | 2.91 g/g | 67 |
| TPT-DHBDX COF | I | TPT-CHO, DHBD | 3.43 | - | 348 | 5.43 g/g(X=0) | 68 |
| SCU-COF-1 | TcO4-/ReO4- | aminated viologen,Tp | 1.44 | - | 373 | 702.4 mg/g | 69 |
| DhaTGCl | TcO4-/ReO4- | Dha, TGCl | 1.5 | 3~12 | 298 | 437 mg/g | 70 |
| PQA-Py-I, PQA- pNH2Py-I, PQA-pN(Me)2Py-I | TcO4-/ReO4- | - | - | - | - | 997 mg/g | 70 |
| CPF-D, CPF-T | U | HCCP, hydroquinone/ phloroglucinol | - | 1 | 298 | 140 mg/g (CPF-T) | 58 |
| MPCOF | U | HCCP, PDA | 1.8 | 4.5 | - | 169 mg/g | 57 |
| 1~2.5 | - | 95 mg/g | |||||
| COF-HBI | U | TMC, PDA, HBI | - | 4.5 | - | 211 mg/g | 72 |
| 4.5 | - | 81 mg/g | |||||
| COF-TpDb-AO | U | Db, Tp, hydroxylamine | 1.58 | - | - | 127 mg/g | 73 |
| PAF-1-CH2AO | U | PAF-1, HCl, NaCN, NH2·OH | 0.7 | 6 | 298 | 300 mg/g | 74 |
| [NH4]+[COF-SO4-] | U | COF-SO3H, NH3·H2O | 1.1 | 5 | 298 | 851 mg/g | 75 |
| 5 | 298 | 17.8 mg/g | |||||
| o-GS-COF | U | TDCOF, QG | - | - | - | 220.1 mg/g | 76 |
| MIPAFs | U | - | - | 6.5 | - | 37.28 mg/g | 77 |
3.2 有机物类
3.3 气体
表2 COFs材料去除有机物污染物Table 2 Applications of diverse COFs in removing organic pollutants |
| COFs | Removal | Building units | Pore size/nm | Conditions | Adsorption capacity | Catalytic amount | ref | ||
|---|---|---|---|---|---|---|---|---|---|
| pH | T/K | ||||||||
| TS-COF-1 | MB | TAPT, PMDA/Tp | 3.3 | - | 298 | 1691 mg/g | - | 84 | |
| TS-COF-2 | 1.5 | - | 298 | 377 mg/g | - | ||||
| Fe-TiO2@COF | MB | TpTa-COF, TiO2, Fe3+ | 2.3 | - | - | - | 100 mg/L,4 mL | 85 | |
| Ag@TPHH-COF | 4-nitrop-henol, NACs | TPT-CHO, hydrazine hydrate, Ag+ | 2.4 | - | 298 | - | - | 86 | |
| g-C3N4@COF | Acid Orange Ⅱ | g-C3N4, TpPa-1 | - | 5.88 | 298 | - | - | 87 | |
| COF1 | TPhP | Tp, Pa-1/BD/DT | 1.81 | 7.5 | 300 | 86.1 mg/g | - | 88 | |
| COF2 | 2.57 | 7.5 | 300 | 387.2 mg/g | - | ||||
| COF3 | 3.34 | 7.5 | 300 | 371.2 mg/g | - | ||||
表3 COFs材料去除气体污染物Table 3 Applications of diverse COFs in removing gaseous pollutants |
| COFs | Removal | Building units | Pore size/nm | Conditions | Adsorption capacity | ref | |
|---|---|---|---|---|---|---|---|
| pH | T/K | ||||||
| COF-10 | NH3 | Hexahydroxytriphenylene, biphenyldiboronic acid | 3.4 | - | 298 | 15 mol/kg | 88 |
| [HOOC]X-COF | NH3 | TP, PA, 2,5-diaminobenzoic acid | 2.4 | - | 298 | 9.34 mmol/g(X=17) | 89 |
| COF-105 | SO2 | TBPS, 2,3,6,7,10,11-hexahydroxy triphenylene | - | - | - | - | 90 |
| PI-COF-mX | SO2 | DMMA, TAPA, PMDA | 2.9 | - | - | 6.30 mmol SO2 g-1 (X=10) | 91 |
| CTF-HUST-HC1 | NO | Terephthalaldehyde, Terephthalamidine dihydrochloride | 1.2 | - | - | - | 92 |