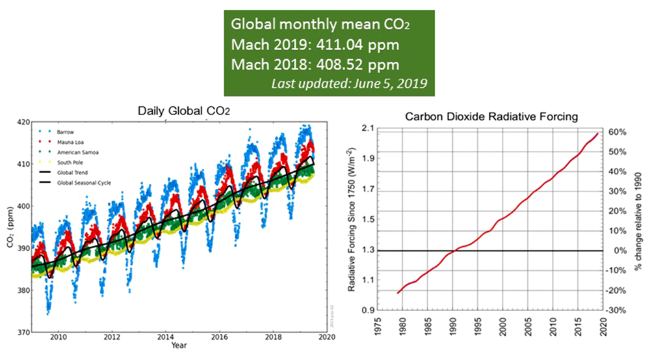
1 引言
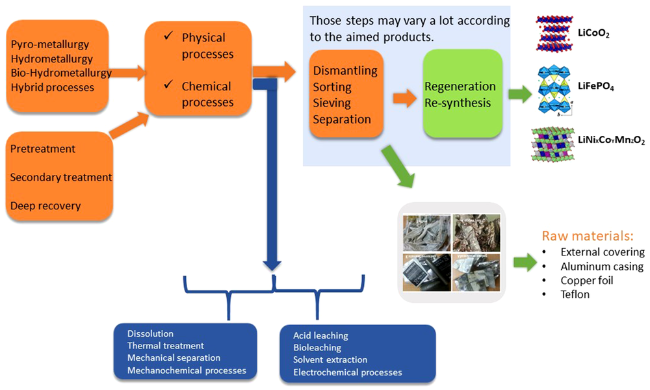
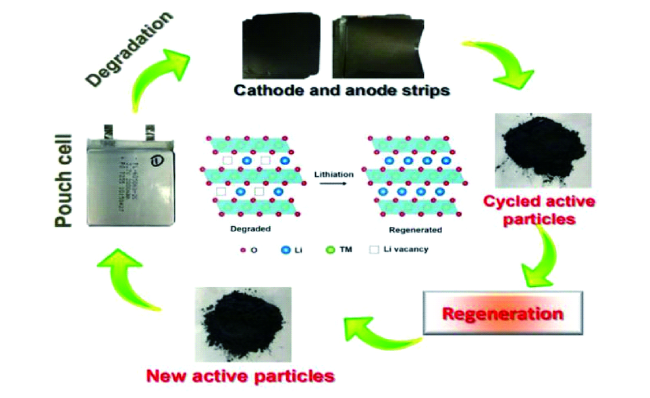
2 废旧锂离子电池回收概述
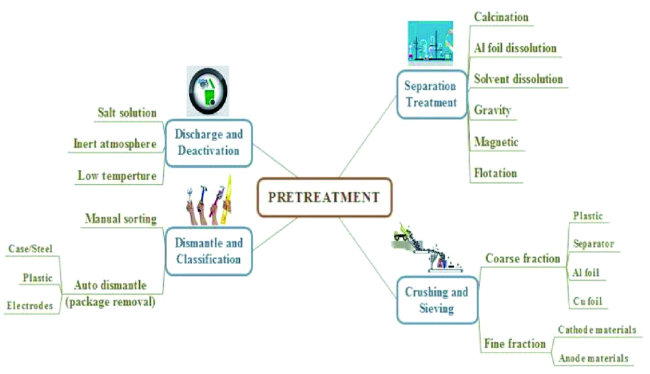
3 废旧锂离子电池的预处理
3.1 放电失活
3.2 分拣拆解
3.3 粉碎筛分
3.4 分离处理
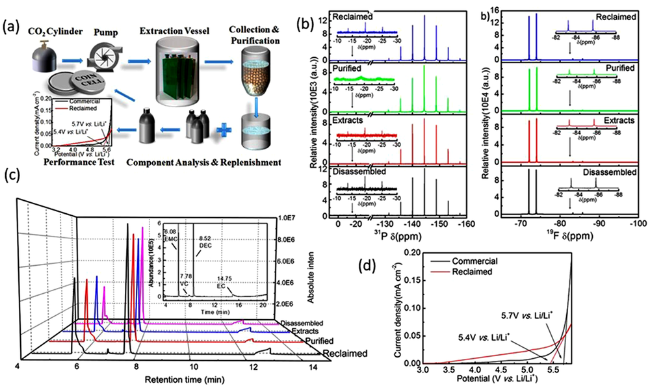
4 废旧电池材料的溶解与纯化
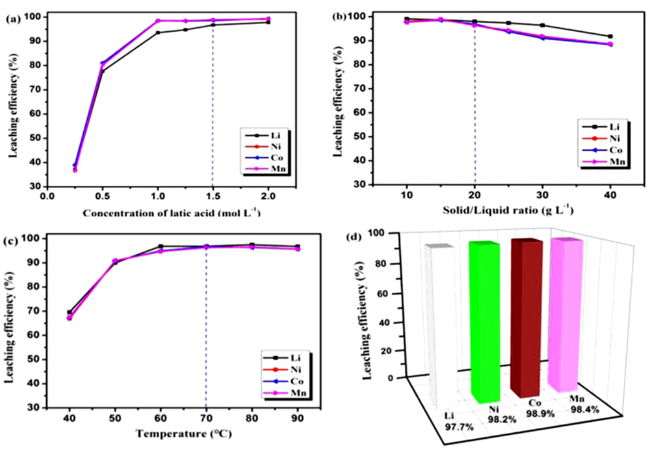
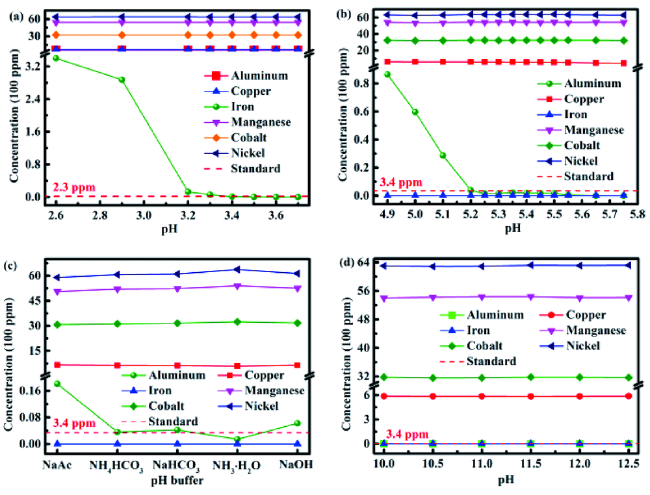
4.1 酸浸溶解
表1 近三年利用有机和无机酸浸出废旧锂离子电池的代表性研究工作Table 1 Typical research works about the inorganic and organic acid leaching for spent lithium-ion batteries in recent years |
| work group | raw material | reagent | leaching conditions | leaching efficiency | ref |
|---|---|---|---|---|---|
| Barik S P(2017) | Spent LIBs | 1.75 mol/L HCl | 50 ℃, 120 min | 99.2%Li, 98%Co, 99%Mn | 22 |
| He L P(2017) | Spent LIBs (LiNi1/3Co1/3Mn1/3O2) | 1 mol/L H2SO4 + 1 vol% H2O2 | 40 ℃, 60 min | 99.7%Li, 99.7%Co 99.7%Mn, 99.7%Ni | 38 |
| Chen X P(2018) | Spent LIBs(LiCoO2) | 1 mol/L H2SO4 + 0.4 g/g Glucose | 95 ℃, 120 min | 96%Li, 98%Co | 39 |
| Guan J(2017) | Spent LIBs | 1 mol/L HNO3 | 250 min(grinding)+ 550 rpm(rotation) | 77.15% Li, 91.25%Co 100%Mn, 99.9%Ni | 40 |
| Chen X P(2017) | Spent LIBs(LiCoO2) | 0.7 mol/L H3PO4 + 4 vol% H2O2 | 40 ℃, 60 min | 99%Li, 99%Co | 41 |
| Meng Q(2017) | Spent LIBs(LiCoO2) | 1.5 mol/L H3PO4 + 0.02 mol/L Glucose | 80 ℃, 120 min | 100%Li, 98%Co | 42 |
| Li L(2019) | Spent LIBs(LiFePO4) | 20 g/g Citric Acid + H2O | 460 min(grinding) +300 rpm(rotation) | 97.8%Li, 95.6%Fe | 43 |
| Yu M(2019) | LiCoO2 | 1.0 mol/L Citric Acid + 8 vol% H2O2 | 70 ℃, 70 min | 99% | 44 |
| Gao W F(2018) | Spent LIBs (LiNi x Co y Mn1- x - y O2) | 3.5 mol/L Acetic Acid + 4 vol% H2O2 | 60 ℃, 60 min | 99.97%Li, 93.62%Co 96.32% Mn, 92.67% Ni | 45 |
| He L P(2017) | Spent LIBs(LiCoO2 and LiNi0.5Co0.2Mn0.3O2) | 2 mol/L L-tartaric Acid + 4 vol% H2O2 | 70 ℃, 30 min | 99.1%Li, 98.6%Co 99.3%Mn, 99.3%Ni | 46 |
| Zhuang L Q (2019) | Spent LIBs (LiNi0.5Co0.2Mn0.3O2) | 0.2 M Phosphoric acid +0.4 M Citric acid | 90 ℃, 30 min | 100% Li, 91.63%Co 92.00% Mn, 93.38%Ni | 47 |