Contents
1 引言
表1 常用的单原子催化剂制备方法Table 1 Commonly used preparation methods for single atom catalysts |
| Preparation method | Catalyst | Carrier | Reaction catalyzed | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|---|
| Coprecipitation | Pt | FeO x | CO oxidation | even distribution of active single atoms on carrier | catalytic activity susceptible to many factors and low load | 17 |
| Ir | FeO x | water gas conversion | 8 | |||
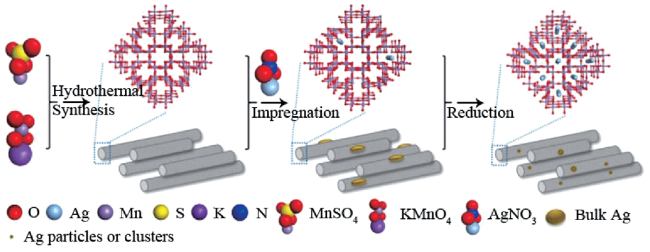
| Ag | Hollandite-type MnO2 | Hydrogenation of glyoxylate | 35 | |||
| 34 | ||||||
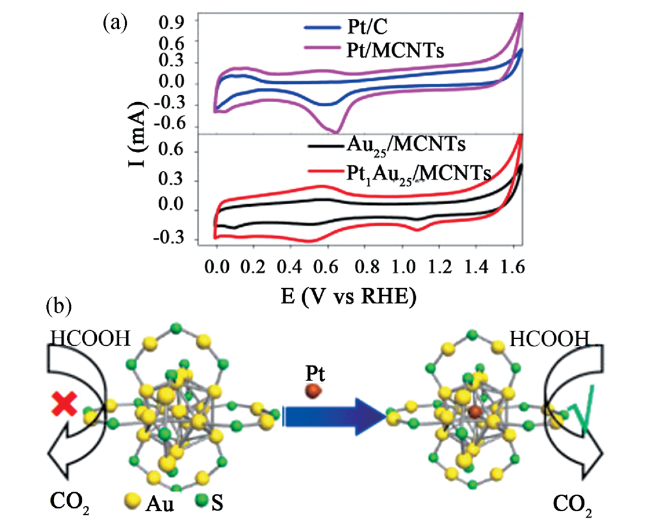
| PtAu | MCNTs | formic acid oxidation | ||||
| Successive reduction method | Au | Pd nanocluster | glucose oxidation | excellent catalytic activity and long shelf life | complicated preparation and hard-to-control structure | 30 |
| Au | IrPd nanocluster | glucose oxidation | 36 | |||
| Au | Pd nanocluster | glucose oxidation | 37 | |||
| Pd1 | Au33or Au43 | benzyl alcohol oxidation | 31 | |||
| NiCu | SiO2 | ethanol dehydrogenation | 38 | |||
| Wet-impregmation method | Pt | Fe-N-C | ORR | facile process and no need for specific appaaratus | Low load | 39 |
| Rh | ZnO | Hydroformylation of olefins | 40 | |||
| Pt | Sb-doped tin oxide | formic acid oxidation | 41 | |||
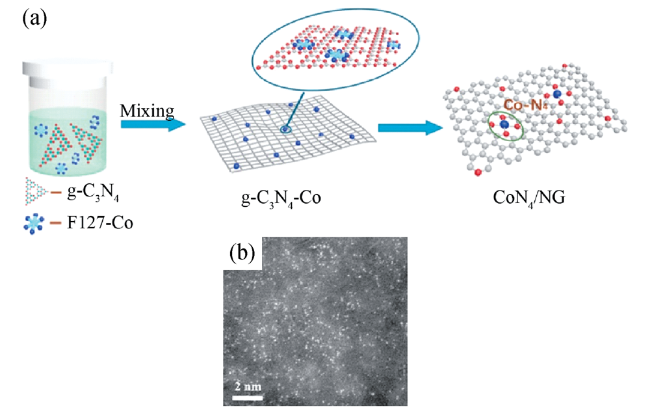
| CoN | graphene | Cathode catalyst of Zn air battery | 42 | |||
| Pt | θ-Al2O3 | CO oxidation | 43 | |||
| Ni | graphene | Electrocatalytic hydrogen evolution | 44 | |||
| Au | TiO2 | water gas conversion | 45 |