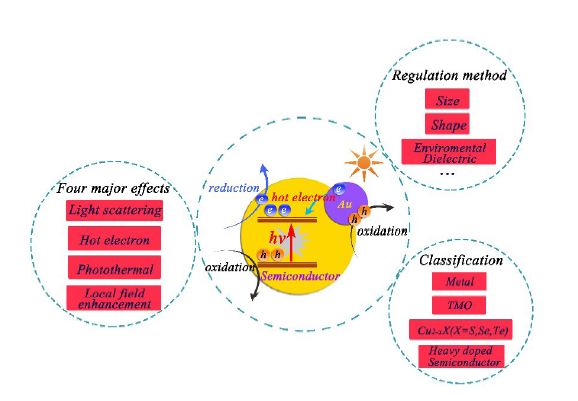
Contents
1 引言
2 表面等离子体效应增强光催化性能的机理
2.1 局域表面等离子体共振产生的机制
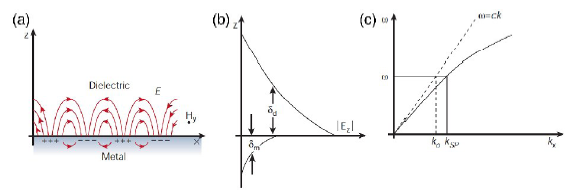
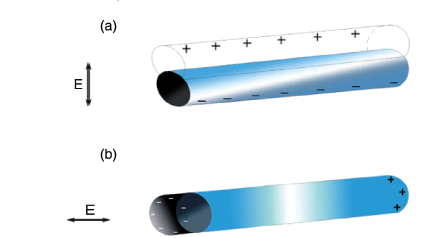
图1 表面等离子激元机理图:(a)SPP机理图;(b)SPP的电场在垂直方向呈指数递减;(c)SPP的色散曲线[40]Fig. 1 The principle of surface plasmons polaritions.(a) SPP mechanism diagram;(b) the electric field of SPP decreases exponentially in the vertical direction;(c) dispersion curve of Surface Plasmon Polariton [40]. Copyright 2003, Springer Nature. |
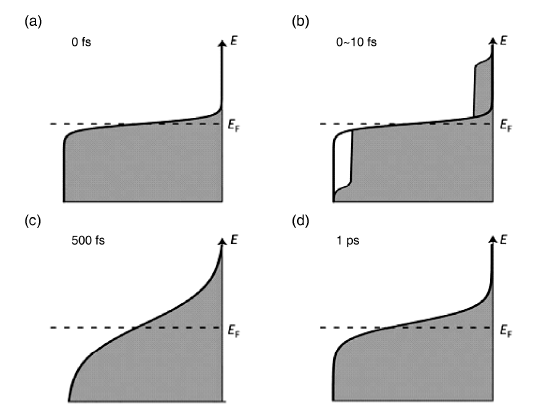
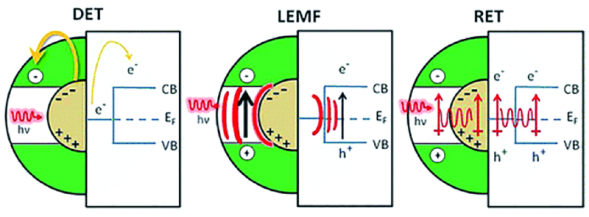
2.2 局域表面等离子体共振的四大效应
2.3 局域表面等离子体共振效应的影响因素
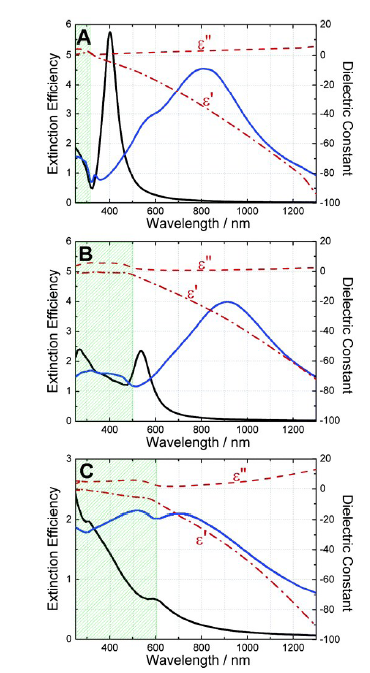
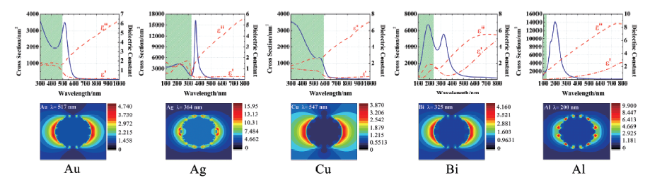
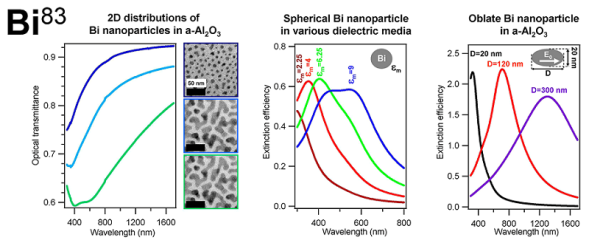
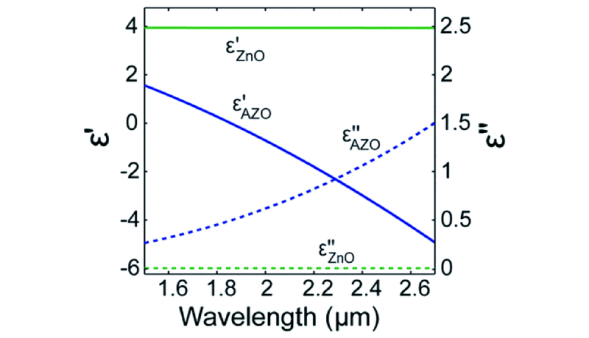
图6 理论计算的纳米球(黑实线)和纳米壳(蓝实线)的远场消光效率:(A)Ag;(B)Au;(C)Cu;ε'和ε″分别为材料介电函数的实部与虚部[61]Fig. 6 Theoretically calculate the far-field extinction efficiency of nanospheres(black solid lines) and nanoshells(blue solid lines):(A) Ag;(B) Au;(C) Cu; ε'and ε″ are the real and imaginary parts of material dielectric functions, respectively [61]. Copyright 2005, American Chemical Society. |
图8 在非相互作用的情况下纳米球电场强度的空间分布:(a)Drude模型;(b)洛伦兹模型;(c~e)Drude-洛伦兹模型在强相互作用情况下;(f)Drude(红线),洛伦兹(绿线)和DL模型(黑线)描述的纳米球的光吸收谱[62]Fig. 8 Spatial distributions of the E-field intensity(|E|2) of a nanosphere(a=10 nm) described by(a) the Drude model and(b) the Lorentz model in the noninteracting cases as well as(c~e) the DL model in the strong interaction case.(f) Optical absorption spectra of the nanospheres described by the Drude(red line), Lorentz(green line), and DL models(dark line)[62]. Copyright 2011, American Chemical Society. |
3 四类表面等离子体光催化体系及其应用
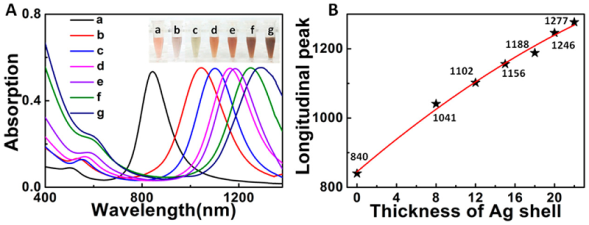
3.1 贵金属基表面等离子体光催化剂
图10 具有不同不连续Ag壳厚度的双金属Au/Ag核-壳超结构的(A)代表性UV-vis-NIR吸收光谱:(a)8,(b)12,(c)15,(d)18,(e) 20,和(f)22 nm;插图为相应的溶液颜色照片。(B)纵向吸收峰强度与Ag壳厚度的关系[82]Fig. 10 Representative UV-vis-NIR absorption spectra of bimetallic Au/Ag core-shell superstructures;(A) with different discontinuous Ag shell thicknesses:(a) 8,(b) 12,(c) 15,(d) 18,(e) 20, and(f) 22 nm; corresponding inset photographs of solution color.(B) Plot of longitudinal absorption peak intensity versus thickness of Ag shell[82]. Copyright 2017, American Chemical Society. |
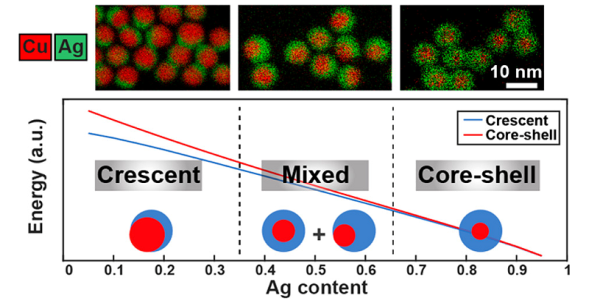
图11 以新月结构是平衡结构的条件下,对于Ag不同原子分数计算三种可能的Cu-Ag形状的能量,这种划分与实验结果相匹配[86]Fig. 11 Calculated energies of three possible Cu-Ag shapes for different atomic fractions of Ag under the condition where the crescent is the equilibrium structure, such division matches the experimentally obtained results [86]. Copyright 2018, American Chemical Society. |
3.2 非贵金属基表面等离子体光催化剂
3.3 缺陷半导体表面等离子体光催化剂
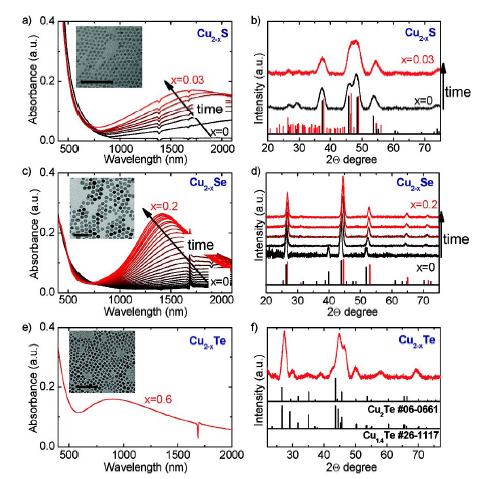
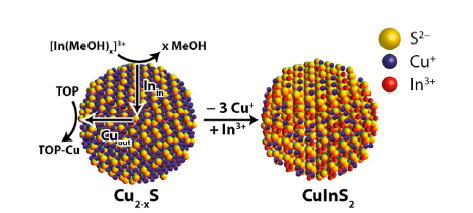
图14 空气暴露氧化过程中化学计量在甲苯中的可见-近红外消光谱的时间演变图和XRD图谱的演变。 (a、c、e)分别为Cu2-xS(x=0,黑色曲线)、Cu2-xSe(x=0,黑色曲线)和Cu2-xTe(x> 0)纳米晶体在甲苯中的Vis-NIR消光光谱。(b、d、f)为氧化期间(a、c、e)中所示的Cu2-xS、Cu 2-xSe、Cu2-xTe的XRD图谱的时间演变[116]Fig. 14 The time evolution of the visible-near-infrared spectroscopy of stoichiometry in toluene during air exposure oxidation and the evolution of the XRD patterns. Nanocrystals in Vis-NIR in toluene Extinction spectrum(a) Cu2-xS(x=0, black curve),(c) Cu2-xSe(x=0, black curve) and(e) Cu2-xTe(x > 0). The time evolution of the XRD patterns of(b) Cu2-xS,(d) Cu2-xSe,(f) Cu2-xTe shown in the oxidation period(a, c, e) [116]. Copyright 2012, American Chemical Society. |
3.4 特殊微/纳结构的LSPR效应
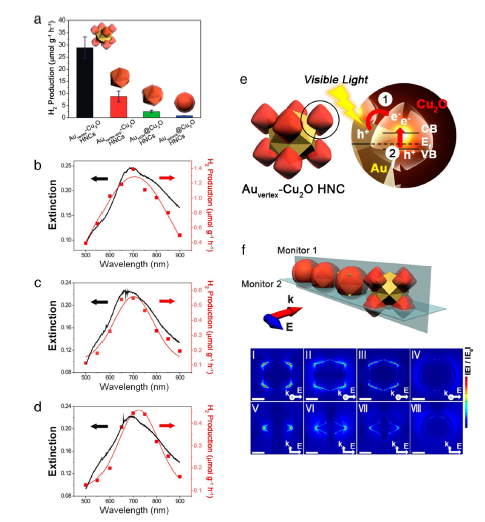
图19 (a)不同异质纳米晶体的光催化氢生成速率。用(b)Auvertex-Cu2O,(c)Auvertex-exp-Cu2O和(d)AuHOH @Cu2O 异质纳米晶体获得的消光光谱;(e)Auvertex-Cu2O 异质纳米晶体可能的等离子体激元诱导的电荷分离过程的示意图;(f)用于计算| E |的FDTD仿真模型异质纳米晶体:Auvertex-Cu2O(Ⅰ,Ⅴ),Auvertex-exp-Cu2O(Ⅱ,Ⅵ),AuHOH @Cu2O(Ⅲ,Ⅶ)和Ausphere @Cu2O异质纳米晶体(Ⅳ,Ⅷ)[148]Fig. 19 (a) Photocatalytic hydrogen generation rate of different heterogeneous nanocrystals; The extinction spectrum obtained with(b) Auvertex-Cu2O,(c) Auvertex-exp-Cu2O and(d) AuHOH@Cu2O heterogeneous nanocrystals.(e) Schematic diagram of possible plasmon-induced charge separation processes for Auvertex-Cu2O heterogeneous nanocrystals.(f) FDTD simulation model heterogeneous nanocrystals used to calculate | E | Auvertex-Cu2O(Ⅰ,Ⅴ), Auvertex-exp-Cu2O(Ⅱ,Ⅵ), AuHOH @Cu2O(Ⅲ,Ⅶ) and Ausphere @Cu2O Heteronanocrystals(Ⅳ,Ⅷ) [148]. Copyright 2016, American Chemical Society. |