 PDF(23293 KB)
PDF(23293 KB)


高电压锂离子正极材料LiNi0.5Mn1.5O4高温特性
高金伙, 阮佳锋, 庞越鹏, 孙皓, 杨俊和, 郑时有
化学进展 ›› 2021, Vol. 33 ›› Issue (8) : 1390-1403.
 PDF(23293 KB)
PDF(23293 KB)
 PDF(23293 KB)
PDF(23293 KB)
高电压锂离子正极材料LiNi0.5Mn1.5O4高温特性
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}High Temperature Properties of LiNi0.5Mn1.5O4 as Cathode Materials for High Voltage Lithium-Ion Batteries
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}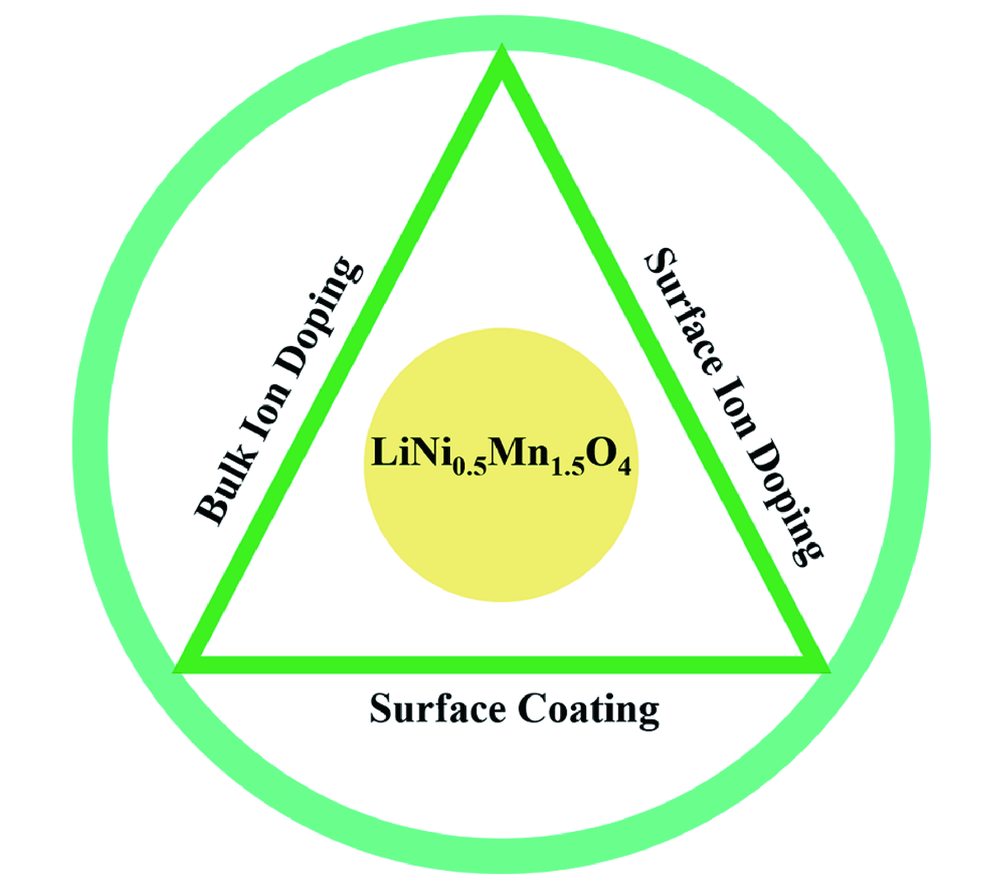
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |