 PDF(4311 KB)
PDF(4311 KB)


 PDF(4311 KB)
PDF(4311 KB)
 PDF(4311 KB)
PDF(4311 KB)
光催化降解土壤中多环芳烃
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Photocatalytic Degradation of Polycyclic Aromatic Hydrocarbon in Soil
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}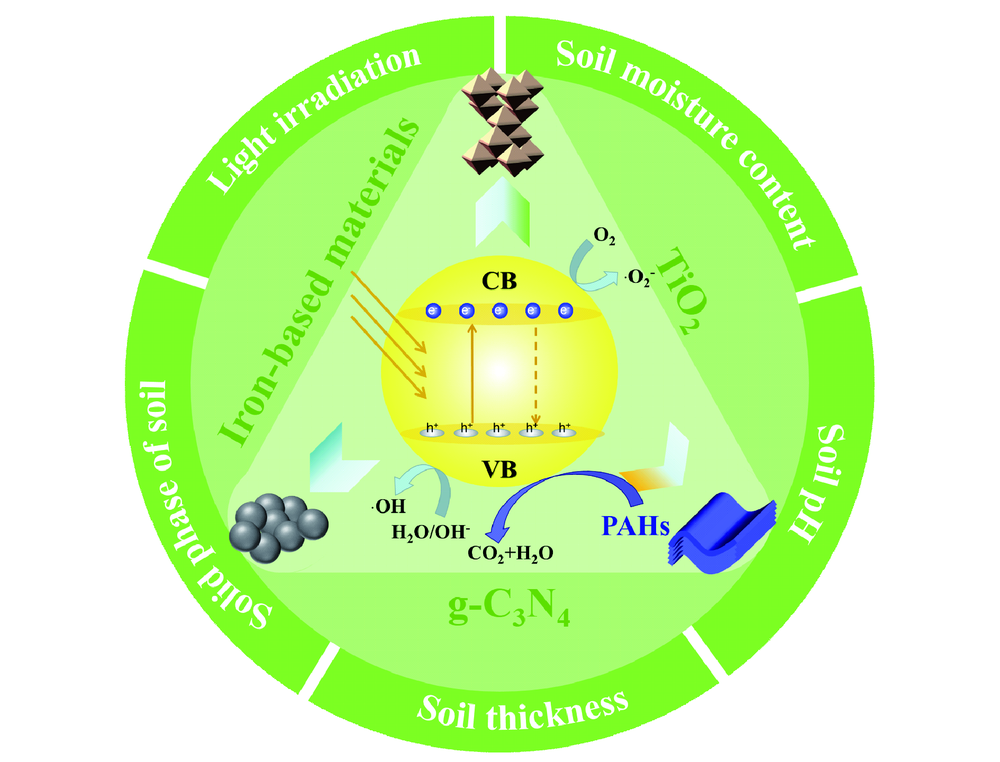
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |