 PDF(2028 KB)
PDF(2028 KB)


 PDF(2028 KB)
PDF(2028 KB)
 PDF(2028 KB)
PDF(2028 KB)
非对称氧杂环丁烷的选择性开环
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Selective Ring-Opening reactions of Unsymmetric Oxetanes
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}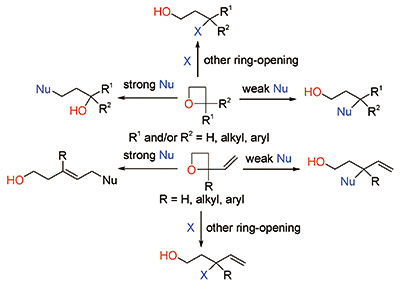
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |