 PDF(7093 KB)
PDF(7093 KB)


 PDF(7093 KB)
PDF(7093 KB)
 PDF(7093 KB)
PDF(7093 KB)
催化氧化技术在可挥发性有机物处理的研究
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Removal of Volatile Organic Compounds by Catalytic Oxidation Technology
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}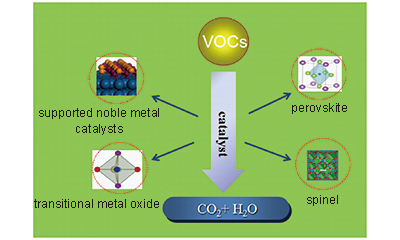
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |