

文章编号: 20190601
文献标识码: A
纳米金属有机框架材料在药物递送领域的应用
收稿日期:2018-10-22
要求修回日期:2019-01-18
网络出版日期:2019-04-26
基金资助
国家自然科学基金项目(51503230)
国家自然科学基金项目(81471778)
国家自然科学基金项目(51203177)
广东省引进创新创业团队项目(2013S086)
广州市科技计划项目(201804010101)
版权
Nanoscale Metal Organic Frameworks for Drug Delivery
Received:22 Oct. 2018
rev-requestrev-request:18 Jan. 2019
Online:26 Apr. 2019
Fund
National Natural Science Foundation of China(51503230)
National Natural Science Foundation of China(81471778)
National Natural Science Foundation of China(51203177)
Guangdong Innovative and Entrepreneurial Research Team Program(2013S086)
Science and Technology Program of Guangzhou,China(201804010101)
Copyright
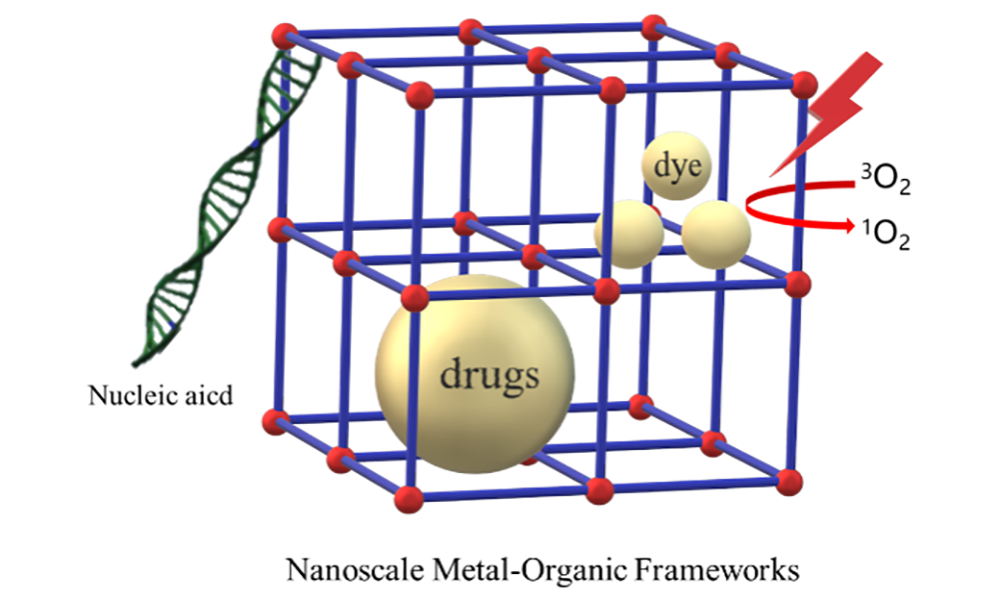
金属有机框架材料(Metal-Organic Frameworks, MOFs)是一类由金属离子及有机配体自组装而成的多孔材料,具有孔隙率高、比表面积大和结构多样化等独特优点,广泛应用于气体储存、物质分离和催化等领域。纳米尺寸金属有机框架材料(Nanoscale Metal-Organic Frameworks, NMOFs)既保持了传统MOFs的规整性,也具有纳米颗粒的特殊性质,在生物医药领域中是绝佳的药物载体。相比于传统纳米药物载体,NMOFs与药物的结合方式丰富,展现了多种药物装载模式,可以满足不同药物的制备需求,也可引入不同功能分子优化性能。最近,有越来越多的研究报道了多功能化NMOFs应用于药物递送领域,并实现刺激响应性的可控释放。本文将着重对NMOFs材料作为药物载体负载抗癌药物、光敏剂和核酸的应用进展进行综述。
赖欣宜 , 王志勇 , 郑永太 , 陈永明 . 纳米金属有机框架材料在药物递送领域的应用[J]. 化学进展, 2020 , 31(6) : 783 -790 . DOI: 10.7536/PC181029
Xinyi Lai , Zhiyong Wang , Yongtai Zheng , Yongming Chen . Nanoscale Metal Organic Frameworks for Drug Delivery[J]. Progress in Chemistry, 2020 , 31(6) : 783 -790 . DOI: 10.7536/PC181029
Metal-organic frameworks(MOFs), a class of self-assembled porous materials with metal ions and organic ligands, have attracted increasing research attention owing to their high porosity, tunable pore size, large surface area and multiple structures. In recent years, MOFs have been extensively investigated in gas storage, separation, catalysis and other fields. When the size of these hybrid materials drops down to nanosized scale, the regular morphology and unique properties make NMOFs become promising candidates for drug delivery. Compared to other nanocarriers, NMOFs provide multiple binding sites for a variety of small-molecule drugs and biomacromolecule via inclusion or surface conjugation. These chemical modifications do not affect NMOFs' intrinsic physicochemical properties. Moreover, the facile synthesis and mild preparation conditions endow NMOFs with advantages in biomedicine. Nowadays, NMOFs have been demonstrated with multifunctionalities and stimuli-responsive controlled release in vivo. Therefore, a detailed review of the application of NMOFs in controlled drug delivery of anticancer drugs, photosensitizer and nucleic acids is provided here.
Key words: metal-organic frameworks(MOFs) ; nanoparticle ; drug delivery ; anticancer drug ; photosensitizer ; nucleic acid
图2 PDA-PCM@ZIF-8/DOX合成路线及可控热疗与化疗示意图。(a) 光照条件下不同pH环境中PDA-PCM@ZIF-8/DOX阿霉素释放曲线;(b) 由近红外光引发的PDA@ZIF-8/DOX及PDA-PCM@ZIF-8/DOX在相同pH条件下的阿霉素释放曲线区别[23]Fig. 2 The synthesis of PDA-PCM@ZIF-8/DOX and controllable combined thermo-chemotherapy.(a) Controlled DOX release behaviors of the PDA-PCM@ZIF-8/DOX at different pH under NIR irradiation.(b) Controlled DOX release behaviors of the PDA@ZIF-8/DOX and PDA-PCM@ZIF-8/DOX at same pH under 37 ℃ shaking with additional 5 min NIR irradiation or not[23]. Copyright 2018, Elsevier |
图3 PCN-224结构示意图:(a) Zr6金属簇、H2TCPP配体及PCN-224的3D纳米孔洞结构;(b) PCN-224立方体单元,及其组成的不同尺寸球形纳米颗粒示意图;(c)90 nm-PCN-224在不同激光条件下杀伤HeLa细胞效果对比;(d)1/4FA-PCN-224与PCN-224体外光动力治疗效果区别[30]Fig. 3 Illustration of PCN-224 structure.(a) six-connected Zr6 cluster(Zr6O4(OH)4(H2O)6(OH)6(COO)6), tetratopic linker(tetrakis(4-carboxyphenyl)porphyrin(H2TCPP)), and 3D nanoporous framework of PCN-224.(b) A cubic unit of PCN-224 and schematic illustration of spherical PCN-224 nanoparticles on the basis of construction of cubic units, yielding different sizes.(c) Control experiments of cytotoxicity in HeLa cells upon light irradiation of 420 and 630 nm in the absence and presence of 90 nm-PCN-224. Irradiation time=30 min.(d) Comparison of in vitro PDT efficacy of pristine 1/4FA-PCN-224and PCN-224 in HeLa cells[30]. Copyright 2016, American Chemical Society |
图6 UiO-66-N3纳米颗粒复合DNA流程示意图:(A) UiO-66-N3合成路线;(B) DBCO功能化DNA与UiO-66-N3的复合;(C) 菌株催化下MOF配体与DNA的点击反应[43]Fig. 6 Synthesis and DNA Functionalization of UiO-66-N3 Nanoparticles.(A) Synthesis of UiO-66-N3(Zr6O4OH4(C8H3O4-N3)6) nano-particles.(B) DNA functionalization of UiO-66-N3 nanoparticles, utilizing DNA functionalized with DBCO.(C) Strain promoted click reaction between a MOF strut and DNA. Zirconium atoms=blue; oxygen atoms=red; carbon atoms=black; azide groups=green. Hydrogen atoms are omitted for clarity[43]. Copyright 2014, American Chemical Society |
图7 ssDNA和MOF之间相互作用调控:(a) ssDNA负载在MOF精确可控孔径中的示意图;(b) 随MOF孔径增大,其与ssDNA间作用力逐渐增强,相对较弱的作用力既保证了ssDNA的高效负载和有效保护,又可实现其可逆释放[44]Fig. 7 Fine-tuning of interactions between ssDNA and MOFs.(a) Illustration of ssDNA inclusion in MOFs composed of bio-compatible organic linkers and with precisely controlled pore sizes.(b) Gradual increase of interaction between ssDNA and MOFs as the pore size of MOF extended progressively. Relatively mild interactions guarantee the uptake and protection of ssDNA in the MOF pores, and also provide reversibility for their release[44]. Copyright 2018, Nature Publishing Group |
| [1] |
Della R J, Liu D, Lin W . J. Shanghai Norm. Univ., 2012,44(10):957.
|
| [2] |
Yaghi O M, Li H, Davis C, Richardson D, Groy T L . Acc. Chem. Res., 1998,31(8):474.
|
| [3] |
Li H, Eddaoudi M, O’Keeffe M, Yaghi O M . Nature, 1999,402(6759):276.
|
| [4] |
Furukawa H, Yaghi O M . ChemInform, 2013,44(45):974.
|
| [5] |
Chen B, Xiang S, Qian G . Acc. Chem. Res., 2010,43(8):1115. https://www.ncbi.nlm.nih.gov/pubmed/20450174
DOI: 10.1021/ar100023y PMID: 20450174 Molecular recognition, an important process in biological and chemical systems, governs the diverse functions of a variety of enzymes and unique properties of some synthetic receptors. Because molecular recognition is based on weak interactions between receptors and substrates, the design and assembly of synthetic receptors to mimic biological systems and the development of novel materials to discriminate different substrates for selective recognition of specific molecules has proved challenging. The extensive research on synthetic receptors for molecular recognition, particularly on noncovalent complexes self-assembled by hydrogen bonding and metal-organic coordination, has revealed some underlying principles. In particular, these studies have demonstrated that the shapes of the supramolecular receptors play significant roles in their specific and selective recognition of substrates: receptors can offer concave surfaces that complement their convex targets. This Account describes our research to develop a synthetic molecular recognition platform using porous metal-organic frameworks (MOFs). These materials contain functional pores to direct their specific and unique recognition of small molecules through several types of interactions: van der Waals interactions of the framework surface with the substrate, metal-substrate interactions, and hydrogen bonding of the framework surface with the substrate. These materials have potential applications for gas storage, separation, and sensing. We demonstrate a simple strategy to construct a primitive cubic net of interpenetrated microporous MOFs from the self-assembly of the paddle-wheel clusters M(2)(CO(2))(4) (M = Cu(2+), Zn(2+), and Co(2+)) with two types of organic dicarboxylic acid and pillar bidentate linkers. This efficient method allows us to rationally tune the micropores to size-exclusively sort different small gas molecules, leading to the highly selective separation and purification of gases. By optimizing the strong interactions between open metal sites within porous MOFs and gas molecules such as hydrogen and acetylene, we have developed several MOF materials with extraordinary acetylene storage capacity at room temperature. We have also immobilized Lewis acidic and basic sites into luminescent porous MOFs to recognize and sense neutral and ionic species. Using the strategy to systematically immobilize different open metal sites within porous MOFs from the metalloligand precursors, we have developed the first microporous mixed-metal-organic framework (M'MOF) with enhanced affinity for hydrogen molecules, which successfully separated D(2) from H(2) using kinetic isotope quantum molecular sieving. Because we can functionalize the pores to direct their specific recognition of small molecules, the emerging porous MOFs serve as novel functional materials for gas storage, separation, heterogeneous catalysis, and sensing. |
| [6] |
Liu J, Chen L, Cui H, Zhang J, Zhang L, Su C Y . Chem. Soc. Rev., 2014,43(16):6011. https://www.ncbi.nlm.nih.gov/pubmed/24871268
DOI: 10.1039/c4cs00094c PMID: 24871268 This review summarizes the use of metal-organic frameworks (MOFs) as a versatile supramolecular platform to develop heterogeneous catalysts for a variety of organic reactions, especially for liquid-phase reactions. Following a background introduction about catalytic relevance to various metal-organic materials, crystal engineering of MOFs, characterization and evaluation methods of MOF catalysis, we categorize catalytic MOFs based on the types of active sites, including coordinatively unsaturated metal sites (CUMs), metalloligands, functional organic sites (FOS), as well as metal nanoparticles (MNPs) embedded in the cavities. Throughout the review, we emphasize the incidental or deliberate formation of active sites, the stability, heterogeneity and shape/size selectivity for MOF catalysis. Finally, we briefly introduce their relevance into photo- and biomimetic catalysis, and compare MOFs with other typical porous solids such as zeolites and mesoporous silica with regard to their different attributes, and provide our view on future trends and developments in MOF-based catalysis. |
| [7] |
Wang C, Liu D, Lin W . J. Am. Chem. Soc., 2013,135(36):13222. https://www.ncbi.nlm.nih.gov/pubmed/23944646
DOI: 10.1021/ja308229p PMID: 23944646 Metal-organic frameworks (MOFs), also known as coordination polymers, represent an interesting class of crystalline molecular materials that are synthesized by combining metal-connecting points and bridging ligands. The modular nature of and mild conditions for MOF synthesis have permitted the rational structural design of numerous MOFs and the incorporation of various functionalities via constituent building blocks. The resulting designer MOFs have shown promise for applications in a number of areas, including gas storage/separation, nonlinear optics/ferroelectricity, catalysis, energy conversion/storage, chemical sensing, biomedical imaging, and drug delivery. The structure-property relationships of MOFs can also be readily established by taking advantage of the knowledge of their detailed atomic structures, which enables fine-tuning of their functionalities for desired applications. Through the combination of molecular synthesis and crystal engineering, MOFs thus present an unprecedented opportunity for the rational and precise design of functional materials. |
| [8] |
Horcajada P, Serre C, Vallet-Regi M, Sebban M, Taulelle F, Ferey G . Angew. Chem.-Int. Ed., 2006,45(36):5974.
|
| [9] |
Davis M E, Chen Z, Shin D M . Nat. Rev. Drug Discov., 2008,7(9):771. https://www.ncbi.nlm.nih.gov/pubmed/18758474
DOI: 10.1038/nrd2614 PMID: 18758474 Nanoparticles--particles in the size range 1-100 nm--are emerging as a class of therapeutics for cancer. Early clinical results suggest that nanoparticle therapeutics can show enhanced efficacy, while simultaneously reducing side effects, owing to properties such as more targeted localization in tumours and active cellular uptake. Here, we highlight the features of nanoparticle therapeutics that distinguish them from previous anticancer therapies, and describe how these features provide the potential for therapeutic effects that are not achievable with other modalities. While large numbers of preclinical studies have been published, the emphasis here is placed on preclinical and clinical studies that are likely to affect clinical investigations and their implications for advancing the treatment of patients with cancer. |
| [10] |
Taylor-Pashow K M L, Della Rocca J, Xie Z, Tran S, Lin W . J. Am. Chem. Soc., 2009,131(40):14261. https://www.ncbi.nlm.nih.gov/pubmed/19807179
DOI: 10.1021/ja906198y PMID: 19807179 Fe(III)-carboxylate nanoscale metal-organic frameworks (NMOFs) with the MIL-101 structure were synthesized using a solvothermal technique with microwave heating. The approximately 200 nm particles were characterized using a variety of methods, including SEM, PXRD, nitrogen adsorption measurements, TGA, and EDX. By replacing a percentage of the bridging ligand (terephthalic acid) with 2-amino terephthalic acid, amine groups were incorporated into the framework to provide sites for covalent attachment of biologically relevant cargoes while still maintaining the MIL-101 structure. In proof-of-concept experiments, an optical contrast agent (a BODIPY dye) and an ethoxysuccinato-cisplatin anticancer prodrug were successfully incorporated into the Fe(III)-carboxylate NMOFs via postsynthetic modifications of the as-synthesized particles. These cargoes are released upon the degradation of the NMOF frameworks, and the rate of cargo release was controlled by coating the NMOF particles with a silica shell. Potential utility of the new NMOF-based nanodelivery vehicles for optical imaging and anticancer therapy was demonstrated in vitro using HT-29 human colon adenocarcinoma cells. |
| [11] |
Liu J, Yang Y, Zhu W, Yi X, Dong Z, Xu X, Chen M, Yang K, Lu G, Jiang L, Liu Z . Biomaterials, 2016,97 1. https://www.ncbi.nlm.nih.gov/pubmed/27155362
DOI: 10.1016/j.biomaterials.2016.04.034 PMID: 27155362 Nanoscale metal organic frameworks (NMOFs) have shown great potential in biomedicine owing to their structural/chemical diversities, high molecular loading capacities, and intrinsic biodegradability. Herein, we report the rational design of a NMOF composed by hafnium (Hf(4+)) and tetrakis (4-carboxyphenyl) porphyrin (TCPP). In such Hf-TCPP NMOFs, while TCPP is a photosensitizer to allow photodynamic therapy (PDT), Hf(4+) with strong X-ray attenuation ability could serve as a radio-sensitizer to enhance radiotherapy (RT). Those NMOFs with polyethylene glycol (PEG) coating show efficient tumor homing upon intravenous injection, and thus could be used for in vivo combined RT & PDT, achieving a remarkable anti-tumor effect. Importantly, Hf-TCPP NMOFs show efficient clearance from the mouse body, minimizing concerns regarding their possible long-term toxicity. Our work thus presents a new concept of developing multifunctional NMOFs as a biodegradable carrier-free system, in which both metal ions and organic ligands are fully utilized to exert their therapeutic functions. |
| [12] |
Lu K, He C, Lin W . J. Am. Chem. Soc., 2014,136(48):16712. https://www.ncbi.nlm.nih.gov/pubmed/25407895
DOI: 10.1021/ja508679h PMID: 25407895 Photodynamic therapy (PDT) is an effective anticancer procedure that relies on tumor localization of a photosensitizer followed by light activation to generate cytotoxic reactive oxygen species (e.g., (1)O2). Here we report the rational design of a Hf-porphyrin nanoscale metal-organic framework, DBP-UiO, as an exceptionally effective photosensitizer for PDT of resistant head and neck cancer. DBP-UiO efficiently generates (1)O2 owing to site isolation of porphyrin ligands, enhanced intersystem crossing by heavy Hf centers, and facile (1)O2 diffusion through porous DBP-UiO nanoplates. Consequently, DBP-UiO displayed greatly enhanced PDT efficacy both in vitro and in vivo, leading to complete tumor eradication in half of the mice receiving a single DBP-UiO dose and a single light exposure. NMOFs thus represent a new class of highly potent PDT agents and hold great promise in treating resistant cancers in the clinic. |
| [13] |
Zhang L, Lei J, Ma F, Ling P, Liu J, Ju H . Chem. Commun., 2015,51(54):10831. https://www.ncbi.nlm.nih.gov/pubmed/26051476
DOI: 10.1039/c5cc03028e PMID: 26051476 A photosensitized and caspase-responsive multifunctional nanoprobe was designed by assembling a porphyrin, a folate targeting-motif and a dye-labelled peptide in a metal-organic framework (MOF) cage, which significantly increases the singlet oxygen quantum yield of porphyrin by 6.2 times, and achieves high efficient cancer therapy and in situ therapeutic monitoring with caspase-3 activation. The integration of theranostic functions in a single nanocarrier holds great promise in precision cancer diagnosis and treatment. |
| [14] |
García-Uriostegui L, Meléndez-Ortiz H I, Toriz G, Delgado E . Mater. Lett., 2017,196 26.
|
| [15] |
Fang Q, Wang J, Gu S, Kaspar R B, Zhuang Z, Zheng J, Guo H, Qiu S, Yan Y . J. Am. Chem. Soc., 2015,137(26):8352. https://www.ncbi.nlm.nih.gov/pubmed/26099722
DOI: 10.1021/jacs.5b04147 PMID: 26099722 Three-dimensional porous crystalline polyimide covalent organic frameworks (termed PI-COFs) have been synthesized. These PI-COFs feature non- or interpenetrated structures that can be obtained by choosing tetrahedral building units of different sizes. Both PI-COFs show high thermal stability (>450 °C) and surface area (up to 2403 m(2) g(-1)). They also show high loading and good release control for drug delivery applications. |
| [16] |
Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, Ferey G, Morris R E, Serre C . Chem. Rev., 2012,112(2):1232. https://www.ncbi.nlm.nih.gov/pubmed/22168547
DOI: 10.1021/cr200256v PMID: 22168547 |
| [17] |
Tamames-Tabar C, Cunha D, Imbuluzqueta E, Ragon F, Serre C, Blanco-Prieto M J, Horcajada P . J. Mater. Chem. B, 2014,2(3):262. https://www.ncbi.nlm.nih.gov/pubmed/32261505
DOI: 10.1039/c3tb20832j PMID: 32261505 2 porosimetry, TEM, DLS and ζ-potential. Their toxicological assessment was performed using two different cell lines: human epithelial cells from foetal cervical carcinoma (HeLa) and murine macrophage cell line (J774). It appears that MOF nanoparticles (NPs) exhibit low cytotoxicity, comparable to those of other commercialised nanoparticulate systems, the less toxic being the Fe carboxylate and the more toxic being the zinc imidazolate NPs. The cytotoxicity values, higher in J774 cells than in HeLa cells, are mainly function of their composition and cell internalisation capacity. Finally, cell uptake of one of the most relevant Fe-MOF-NPs for drug vectorisation has been investigated by confocal microscopy studies, and indicates a faster kinetics of cell penetration within J774 compared to HeLa cells.]]> |
| [18] |
Rieter W J, Pott K M, Taylor K M L, Lin W .J. Am. Chem. Soc., 2008,130(35):11584.
|
| [19] |
Filippousi M, Turner S, Leus K, Siafaka P I, Tseligka E D, Vandichel M, Nanaki S G, Vizirianakis I S, Bikiaris D N, Van Der Voort P, Van Tendeloo G . Int. J. Pharm., 2016,509(1):208.
|
| [20] |
Liu D, He C, Poon C, Lin W . J. Mater. Chem. B, 2014,2(46):8249. https://www.ncbi.nlm.nih.gov/pubmed/32262098
DOI: 10.1039/c4tb00751d PMID: 32262098 2+ ions (13 ± 4 wt%) for potential cancer therapy and magnetic resonance imaging, respectively. The Mn-bisphosphonate NCP was further coated with lipid and pegylated to control the drug release kinetics and functionalized with a targeting group (anisamide) to endow specificity to cancer cells, leading to significantly enhanced cytotoxicity against human breast and pancreatic cancer cells. In vitro MR imaging studies demonstrated the efficacy of the Mn-bisphosphonate NCP as an effective T1 contrast agent and confirmed the targeting capability of anisamide-functionalized NCP. Multifunctional NCPs thus present an excellent platform for designing theranostic nanomaterials for a wide range of biomedical applications.]]> |
| [21] |
Ganta S, Devalapally H, Shahiwala A, Amiji M . J. Controlled Release, 2008,126(3):187. https://linkinghub.elsevier.com/retrieve/pii/S0168365908000254
|
| [22] |
Shenoy D, Little S, Langer R, Amiji M . Pharm. Res., 2005,22(12):2107. https://www.ncbi.nlm.nih.gov/pubmed/16254763
DOI: 10.1007/s11095-005-8343-0 PMID: 16254763 This study was carried out to determine the biodistribution profiles and tumor localization potential of poly(ethylene oxide) (PEO)-modified poly(beta-amino ester) (PbAE) as a novel, pH-sensitive biodegradable polymeric nanoparticulate system for tumor-targeted drug delivery. |
| [23] |
Wu Q, Niu M, Chen X, Tan L, Fu C, Ren X, Ren J, Li L, Xu K, Zhong H, Meng X . Biomaterials, 2018,162:132. https://www.ncbi.nlm.nih.gov/pubmed/29448141
DOI: 10.1016/j.biomaterials.2018.02.022 PMID: 29448141 Zeolitic imidazolate frameworks (ZIFs) have attracted great interest as pH-sensitive drug carrier because of high drug loading and intrinsic biodegradability. In this work, a biocompatible NIR and pH-responsive drug delivery nanoplatform based on ZIFs (PDA-PCM@ZIF-8/DOX) is synthesized for in vivo cancer therapy. The biocompatibility of ZIFs is greatly improved by polydopamine (PDA) modifying and proved by cytotoxicity and in vivo acute toxicity evaluation. The degradability is also regulated in an appropriate rate. Due to mild reaction condition of ZIFs, the synthesis and drug loading is achieved in one pot with high loading (37.86%) and encapsulation rate (78.76%). Meanwhile, PDA acts as a photothermal transfer agent to trigger thermal response switch of phase change materials for NIR controlled drug release. Under the dual stimulus of NIR and acid environment, the drug release is as high as 78%, while only 21% is released without stimulus, showing a remarkable effect of control release. In vivo anti-tumor experiments demonstrate the high tumor inhibition rate of photothermal-chemotherapy group with a significant synergistic effect. The biocompatible and biodegradable drug delivery platform based on ZIFs has shown great promise for future clinic cancer therapy. |
| [24] |
Zheng H, Zhang Y, Liu L, Wan W, Guo P, Nyström A M, Zou X . J. Am. Chem. Soc., 2016,138(3):962. https://www.ncbi.nlm.nih.gov/pubmed/26710234
DOI: 10.1021/jacs.5b11720 PMID: 26710234 Many medical and chemical applications require target molecules to be delivered in a controlled manner at precise locations. Metal-organic frameworks (MOFs) have high porosity, large surface area, and tunable functionality and are promising carriers for such purposes. Current approaches for incorporating target molecules are based on multistep postfunctionalization. Here, we report a novel approach that combines MOF synthesis and molecule encapsulation in a one-pot process. We demonstrate that large drug and dye molecules can be encapsulated in zeolitic imidazolate framework (ZIF) crystals. The molecules are homogeneously distributed within the crystals, and their loadings can be tuned. We show that ZIF-8 crystals loaded with the anticancer drug doxorubicin (DOX) are efficient drug delivery vehicles in cancer therapy using pH-responsive release. Their efficacy on breast cancer cell lines is higher than that of free DOX. Our one-pot process opens new possibilities to construct multifunctional delivery systems for a wide range of applications. |
| [25] |
Dong K, Wang Z, Zhang Y, Ren J, Qu X . ACS Appl. Mater. Interfaces, 2018,10(38):31998.
|
| [26] |
Agostinis P, Berg K, Cengel K A, Foster T H, Girotti A W, Gollnick S O, Hahn S M, Hamblin M R, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson B C, Golab J . CA. Cancer J. Clin., 2011,61(4):250. https://www.ncbi.nlm.nih.gov/pubmed/21617154
DOI: 10.3322/caac.20114 PMID: 21617154 Photodynamic therapy (PDT) is a clinically approved, minimally invasive therapeutic procedure that can exert a selective cytotoxic activity toward malignant cells. The procedure involves administration of a photosensitizing agent followed by irradiation at a wavelength corresponding to an absorbance band of the sensitizer. In the presence of oxygen, a series of events lead to direct tumor cell death, damage to the microvasculature, and induction of a local inflammatory reaction. Clinical studies revealed that PDT can be curative, particularly in early stage tumors. It can prolong survival in patients with inoperable cancers and significantly improve quality of life. Minimal normal tissue toxicity, negligible systemic effects, greatly reduced long-term morbidity, lack of intrinsic or acquired resistance mechanisms, and excellent cosmetic as well as organ function-sparing effects of this treatment make it a valuable therapeutic option for combination treatments. With a number of recent technological improvements, PDT has the potential to become integrated into the mainstream of cancer treatment. |
| [27] |
Bechet D, Couleaud P, Frochot C, Viriot M L, Guillemin F, Barberi-Heyob M . Trends Biotechnol., 2008,26(11):612. https://www.ncbi.nlm.nih.gov/pubmed/18804298
DOI: 10.1016/j.tibtech.2008.07.007 PMID: 18804298 Photodynamic therapy (PDT) in cancer treatment involves the uptake of a photosensitizer by cancer tissue followed by photoirradiation. The use of nanoparticles as carriers of photosensitizers is a very promising approach because these nanomaterials can satisfy all the requirements for an ideal PDT agent. This review describes and compares the different individual types of nanoparticles that are currently in use for PDT applications. Recent advances in the use of nanoparticles, including inorganic oxide-, metallic-, ceramic-, and biodegradable polymer-based nanomaterials as carriers of photosensitizing agents, are highlighted. We describe the nanoparticles in terms of stability, photocytotoxic efficiency, biodistribution and therapeutic efficiency. Finally, we summarize exciting new results concerning the improvement of the photophysical properties of nanoparticles by means of biphotonic absorption and upconversion. |
| [28] |
Lu K, He C, Lin W . J. Am. Chem. Soc., 2015,137(24):7600. https://www.ncbi.nlm.nih.gov/pubmed/26068094
DOI: 10.1021/jacs.5b04069 PMID: 26068094 We report here the rational design of the first chlorin-based nanoscale metal-organic framework (NMOF), DBC-UiO, with much improved photophysical properties over the previously reported porphyrin-based NMOF, DBP-UiO. Reduction of the DBP ligands in DBP-UiO to the DBC ligands in DBC-UiO led to a 13 nm red shift and an 11-fold increase in the extinction coefficient of the lowest-energy Q band. While inheriting the crystallinity, stability, porosity, and nanoplate morphology of DBP-UiO, DBC-UiO sensitizes more efficient (1)O2 generation and exhibits significantly enhanced photodynamic therapy (PDT) efficacy on two colon cancer mouse models as a result of its improved photophysical properties. Both apoptosis and immunogenic cell death contributed to killing of cancer cells in DBC-UiO-induced PDT. |
| [29] |
Sykes E A, Chen J, Zheng G, Chan W C W . ACS Nano, 2014,8(6):5696. https://www.ncbi.nlm.nih.gov/pubmed/24821383
DOI: 10.1021/nn500299p PMID: 24821383 Understanding the principles governing the design of nanoparticles for tumor targeting is essential for the effective diagnosis and treatment of solid tumors. There is currently a poor understanding of how to rationally engineer nanoparticles for tumor targeting. Here, we engineered different-sized spherical gold nanoparticles to discern the effect of particle diameter on passive (poly(ethylene glycol)-coated) and active (transferrin-coated) targeting of MDA-MB-435 orthotopic tumor xenografts. Tumor accumulation of actively targeted nanoparticles was found to be 5 times faster and approximately 2-fold higher relative to their passive counterparts within the 60 nm diameter range. For 15, 30, and 100 nm, we observed no significant differences. We hypothesize that such enhancements are the result of an increased capacity to penetrate into tumors and preferentially associate with cancer cells. We also use computational modeling to explore the mechanistic parameters that can impact tumor accumulation efficacy. We demonstrate that tumor accumulation can be mediated by high nanoparticle avidity and are weakly dependent on their plasma clearance rate. Such findings suggest that empirical models can be used to rapidly screen novel nanomaterials for relative differences in tumor targeting without the need for animal work. Although our findings are specific to MDA-MB-435 tumor xenografts, our experimental and computational findings help to enrich knowledge of design considerations that will aid in the optimal engineering of spherical gold nanoparticles for cancer applications in the future. |
| [30] |
Park J, Jiang Q, Feng D, Mao L, Zhou H C . J. Am. Chem. Soc., 2016,138(10):3518. https://www.ncbi.nlm.nih.gov/pubmed/26894555
DOI: 10.1021/jacs.6b00007 PMID: 26894555 The understanding of nanomaterials for targeted cancer therapy is of great importance as physical parameters of nanomaterials have been shown to be strong determinants that can promote cellular responses. However, there have been rare platforms that can vastly tune the core of nanoparticles at a molecular level despite various nanomaterials employed in such studies. Here we show targeted photodynamic therapy (PDT) with Zr(IV)-based porphyrinic metal-organic framework (MOF) nanoparticles. Through a bottom-up approach, the size of MOF nanoparticles was precisely tuned in a broad range with a designed functional motif, built upon selection of building blocks of the MOF. In particular, molecular properties of the porphyrinic linker are maintained in the MOF nanoparticles regardless of their sizes. Therefore, size-dependent cellular uptake and ensuing PDT allowed for screening of the optimal size of MOF nanoparticles for PDT while MOF nanoparticle formulation of the photosensitizer showed better PDT efficacy than that of its small molecule. Additionally, Zr6 clusters in the MOF enabled an active targeting modality through postsynthetic modification, giving even more enhanced PDT efficacy. Together with our finding of size controllability covering a broad range in the nano regime, we envision that MOFs can be a promising nanoplatform by adopting advanced small molecule systems into the tunable framework with room for postsynthetic modification. |
| [31] |
Allison R R, Sibata C H . Photodiagnosis Photodyn. Ther., 2010,7(2):61. https://www.ncbi.nlm.nih.gov/pubmed/20510301
DOI: 10.1016/j.pdpdt.2010.02.001 PMID: 20510301 A myriad of naturally occurring and synthetic structures are capable of transferring the energy of light. Few, however, allow for this energy transfer to enable a type II photochemical reaction which, as currently practiced, is a fundamental component of photodynamic therapy. Even fewer of these agents, aptly termed photosensitizers, have found success in the treatment of patients. This review will focus on the oncologic photosensitizers that have come to clinical trial with outcomes published in peer reviewed journals. Based on a clinical orientation the qualities of successful photosensitizers will be examined, how current drugs fare and potential future options explored. |
| [32] |
Gao S, Zheng P, Li Z, Feng X, Yan W, Chen S, Guo W, Liu D, Yang X, Wang S, Liang X J, Zhang J . Biomaterials, 2018,178:83. https://www.ncbi.nlm.nih.gov/pubmed/29913389
DOI: 10.1016/j.biomaterials.2018.06.007 PMID: 29913389 2 and the circulation lifetime of photosensitizers for photodynamic therapy (PDT) in vivo would be a promising approach to eliminate hypoxic tumors. Herein, by taking advantage of the significant gas-adsorption capability of metal-organic frameworks (MOFs), a biomimetic O2-evolving photodynamic therapy (PDT) nanoplatform with long circulating properties was fabricated. Zirconium (IV)-based MOF (UiO-66) was used as a vehicle for O2 storing, then conjugated with indocyanine green (ICG) by coordination reaction, and further coated with red blood cell (RBC) membranes. Upon 808 nm laser irradiation, the initial singlet oxygen (1O2) generated by ICG would decompose RBC membranes. At the same time, The photothermal property of ICG could facilitate the burst release of O2 from UiO-66. Subsequently, the generated O2 could significantly improve the PDT effects on hypoxic tumor. Owing to the advantages of long circulation and O2 self-sufficient, the designed nanotherapeutic agent can improve the efficiency of treatment against hypoxia tumor via PDT. Hence, this study presents a new paradigm for co-delivery of O2 and photosensitizers, and provides a new avenue to eliminate hypoxic tumors.]]> |
| [33] |
Cheng H, Zhu J Y, Li S Y, Zeng J Y, Lei Q, Chen K W, Zhang C, Zhang X Z . Adv. Funct. Mater., 2016,26(43):7847.
|
| [34] |
Juliano R L . Nucleic Acids Res., 2016,44(14):6518. https://www.ncbi.nlm.nih.gov/pubmed/27084936
DOI: 10.1093/nar/gkw236 PMID: 27084936 The oligonucleotide therapeutics field has seen remarkable progress over the last few years with the approval of the first antisense drug and with promising developments in late stage clinical trials using siRNA or splice switching oligonucleotides. However, effective delivery of oligonucleotides to their intracellular sites of action remains a major issue. This review will describe the biological basis of oligonucleotide delivery including the nature of various tissue barriers and the mechanisms of cellular uptake and intracellular trafficking of oligonucleotides. It will then examine a variety of current approaches for enhancing the delivery of oligonucleotides. This includes molecular scale targeted ligand-oligonucleotide conjugates, lipid- and polymer-based nanoparticles, antibody conjugates and small molecules that improve oligonucleotide delivery. The merits and liabilities of these approaches will be discussed in the context of the underlying basic biology. |
| [35] |
Dowdy S F . Nat. Biotechnol., 2017,35(3):222. https://www.ncbi.nlm.nih.gov/pubmed/28244992
DOI: 10.1038/nbt.3802 PMID: 28244992 RNA-based therapeutics, such as small-interfering (siRNAs), microRNAs (miRNAs), antisense oligonucleotides (ASOs), aptamers, synthetic mRNAs and CRISPR-Cas9, have great potential to target a large part of the currently undruggable genes and gene products and to generate entirely new therapeutic paradigms in disease, ranging from cancer to pandemic influenza to Alzheimer's disease. However, for these RNA modalities to reach their full potential, they first need to overcome a billion years of evolutionary defenses that have kept RNAs on the outside of cells from invading the inside of cells. Overcoming the lipid bilayer to deliver RNA into cells has remained the major problem to solve for widespread development of RNA therapeutics, but recent chemistry advances have begun to penetrate this evolutionary armor. |
| [36] |
Panyam J, Labhasetwar V . Adv. Drug Deliv. Rev., 2003,55(3):329. https://www.ncbi.nlm.nih.gov/pubmed/12628320
DOI: 10.1016/s0169-409x(02)00228-4 PMID: 12628320 Biodegradable nanoparticles formulated from poly (D,L-lactide-co-glycolide) (PLGA) have been extensively investigated for sustained and targeted/localized delivery of different agents including plasmid DNA, proteins and peptides and low molecular weight compounds. Research about the mechanism of intracellular uptake of nanoparticles, their trafficking and sorting into different intracellular compartments, and the mechanism of enhanced therapeutic efficacy of nanoparticle-encapsulated agent at cellular level is more recent and is the primary focus of the review. Recent studies in our laboratory demonstrated rapid escape of PLGA nanoparticles from the endo-lysosomal compartment into cytosol following their uptake. Based on the above mechanism, various potential applications of nanoparticles for delivery of therapeutic agents to the cells and tissue are discussed. |
| [37] |
An J, Geib S J, Rosi N L . J. Am. Chem. Soc., 2009,131(24):8376. https://www.ncbi.nlm.nih.gov/pubmed/19489551
DOI: 10.1021/ja902972w PMID: 19489551 A porous anionic metal-organic framework, bio-MOF-1, constructed using adenine as a biomolecular building block is described. The porosity of this material is evaluated, its stability in biological buffers is studied, and its potential as a material for controlled drug release is investigated. Specifically, procainamide HCl is loaded into the pores of bio-MOF-1 using a simple cation exchange process. Exogenous cations from biological buffers are shown to affect the release of the adsorbed drug molecules. |
| [38] |
He C, Lu K, Liu D, Lin W . J. Am. Chem. Soc., 2014,136(14):5181. https://www.ncbi.nlm.nih.gov/pubmed/24669930
DOI: 10.1021/ja4098862 PMID: 24669930 Ovarian cancer is the leading cause of death among women with gynecological malignancies. Acquired resistance to chemotherapy is a major limitation for ovarian cancer treatment. We report here the first use of nanoscale metal-organic frameworks (NMOFs) for the co-delivery of cisplatin and pooled small interfering RNAs (siRNAs) to enhance therapeutic efficacy by silencing multiple drug resistance (MDR) genes and resensitizing resistant ovarian cancer cells to cisplatin treatment. UiO NMOFs with hexagonal-plate morphologies were loaded with a cisplatin prodrug and MDR gene-silencing siRNAs (Bcl-2, P-glycoprotein [P-gp], and survivin) via encapsulation and surface coordination, respectively. NMOFs protect siRNAs from nuclease degradation, enhance siRNA cellular uptake, and promote siRNA escape from endosomes to silence MDR genes in cisplatin-resistant ovarian cancer cells. Co-delivery of cisplatin and siRNAs with NMOFs led to an order of magnitude enhancement in chemotherapeutic efficacy in vitro, as indicated by cell viability assay, DNA laddering, and Annexin V staining. This work shows that NMOFs hold great promise in the co-delivery of multiple therapeutics for effective treatment of drug-resistant cancers. |
| [39] |
Chen Q, Xu M, Zheng W, Xu T, Deng H, Liu J . ACS Appl. Mater. Interfaces, 2017,9(8):6712. https://pubs.acs.org/doi/10.1021/acsami.6b12792
|
| [40] |
Wang H, Zhang J, Yu H . Free Radic. Biol. Med., 2007,42(10):1524. https://www.ncbi.nlm.nih.gov/pubmed/17448899
DOI: 10.1016/j.freeradbiomed.2007.02.013 PMID: 17448899 Glutathione peroxidase and thioredoxin reductase are major selenoenzymes through which selenium exerts powerful antioxidant effects. Selenium also elicits pro-oxidant effects at toxic levels. The antioxidant and pro-oxidant effects, or bioavailability and toxicity, of selenium depend on its chemical form. Selenomethionine is considered to be the most appropriate supplemental form due to its excellent bioavailability and lower toxicity compared to various selenium compounds. The present studies reveal that, compared with selenomethionine, elemental selenium at nano size (Nano-Se) possesses equal efficacy in increasing the activities of glutathione peroxidase and thioredoxin reductase but has much lower toxicity as indicated by median lethal dose, acute liver injury, and short-term toxicity. Our results suggest that Nano-Se can serve as an antioxidant with reduced risk of selenium toxicity. |
| [41] |
Bergamo A, Gaiddon C, Schellens J H M, Beijnen J H, Sava G .J. Inorg. Biochem., 2012,106(1):90.
|
| [42] |
Wang S, McGuirk C M, Ross M B, Wang S, Chen P, Xing H, Liu Y, Mirkin C A .J. Am. Chem. Soc., 2017,139(29):9827.
|
| [43] |
Morris W, Briley W E, Auyeung E, Cabezas M D, Mirkin C A . J. Am. Chem. Soc., 2014,136(20):7261. https://www.ncbi.nlm.nih.gov/pubmed/24818877
DOI: 10.1021/ja503215w PMID: 24818877 Nanoparticles of a metal-organic framework (MOF), UiO-66-N3 (Zr6O4OH4(C8H3O4-N3)6), were synthesized. The surface of the MOF was covalently functionalized with oligonucleotides, utilizing a strain promoted click reaction between DNA appended with dibenzylcyclooctyne and azide-functionalized UiO-66-N3 to create the first MOF nanoparticle-nucleic acid conjugates. The structure of the framework was preserved throughout the chemical transformation, and the surface coverage of DNA was quantified. Due to the small pore sizes, the particles are only modified on their surfaces. When dispersed in aqueous NaCl, they exhibit increased stability and enhanced cellular uptake when compared with unfunctionalized MOF particles of comparable size. |
| [44] |
Peng S, Bie B, Sun Y, Liu M, Cong H, Zhou W, Xia Y, Tang H, Deng H, Zhou X . Nat. Commun., 2018,9(1):1293. https://www.ncbi.nlm.nih.gov/pubmed/29615605
DOI: 10.1038/s41467-018-03650-w PMID: 29615605 Effective transfection of genetic molecules such as DNA usually relies on vectors that can reversibly uptake and release these molecules, and protect them from digestion by nuclease. Non-viral vectors meeting these requirements are rare due to the lack of specific interactions with DNA. Here, we design a series of four isoreticular metal-organic frameworks (Ni-IRMOF-74-II to -V) with progressively tuned pore size from 2.2 to 4.2 nm to precisely include single-stranded DNA (ssDNA, 11-53 nt), and to achieve reversible interaction between MOFs and ssDNA. The entire nucleic acid chain is completely confined inside the pores providing excellent protection, and the geometric distribution of the confined ssDNA is visualized by X-ray diffraction. Two MOFs in this series exhibit excellent transfection efficiency in mammalian immune cells, 92% in the primary mouse immune cells (CD4+ T cell) and 30% in human immune cells (THP-1 cell), unrivaled by the commercialized agents (Lipo and Neofect). |
/
| 〈 |
|
〉 |