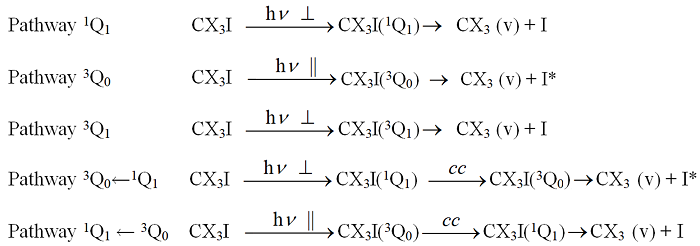1 引言
2 重要小分子的光解离动力学研究
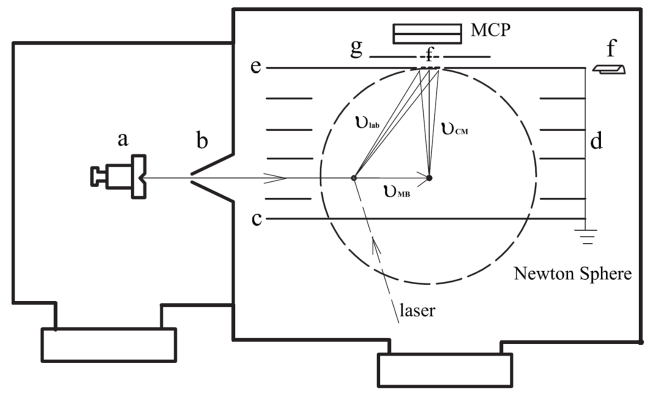
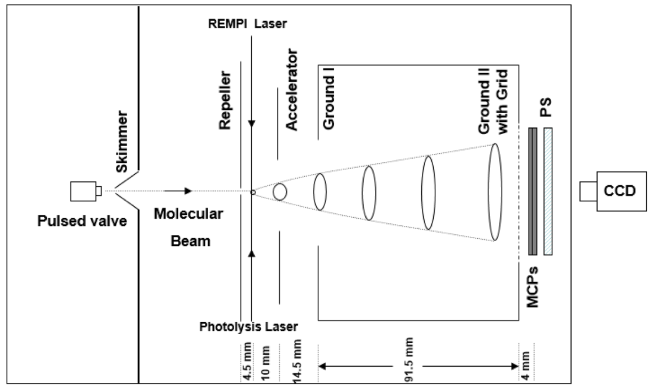
2.1 光解碎片平动能谱仪的研制
2.2 卤代甲烷的光解离研究
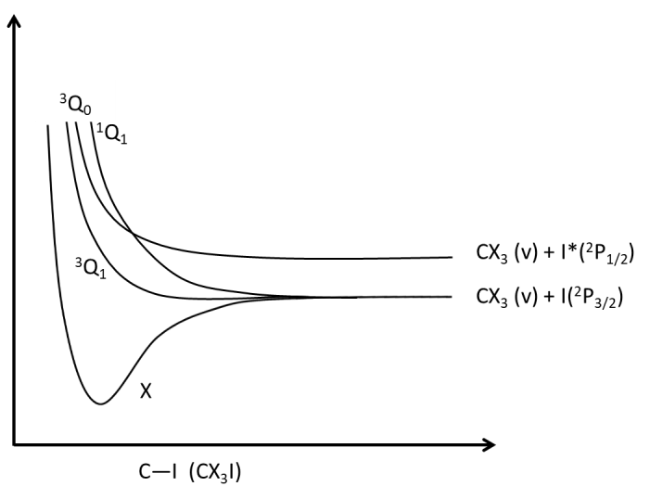
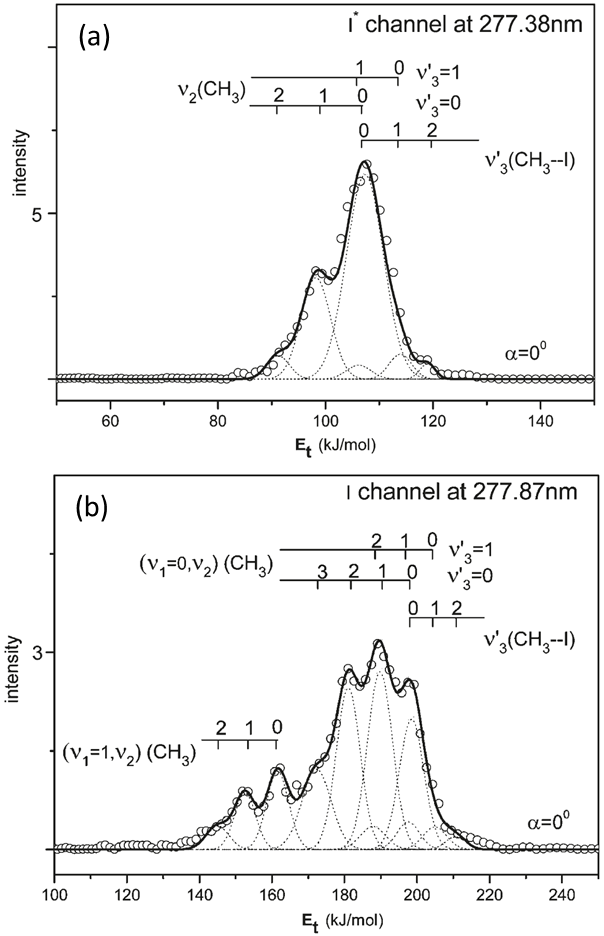
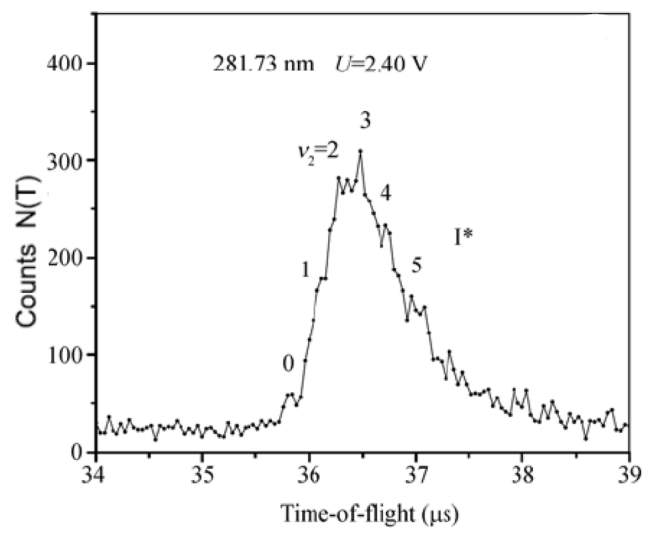
2.2.1 碘甲烷(CH3I)的光解离研究
表1 CH3I光解的动力学参数Table 1 The dynamical parameters for the photodissociation of CH3I |
| λ(nm) | Pathway | Channel | Fraction | Φ(I*) | Pcc | Eint/Eavl | β | Ref. |
|---|---|---|---|---|---|---|---|---|
| 225 | 3Q0 | I* | 0.09 | 0.12 | 0.129 | 1.25 | 43 | |
| 3Q0$\leftarrow$1Q1 | I* | 0.03 | 0.08 | 0.104 | ||||
| 1Q1 | I | 0.34 | 0.134 | 0.84 | ||||
| 1Q1$\leftarrow$3Q0 | I | 0.54 | 0.86 | 0.159 | ||||
| 248 | 3Q0 | I* | 0.74 | 0.74 | 0.26 | 0.125 | 1.85 | 14, 37-39 |
| 1Q1$\leftarrow$3Q0 | I | 0.26 | 0.173 | |||||
| 277 | 3Q0 | I* | 0.59 | 0.59 | 0.028 | 1.93 | 42 | |
| 1Q1$\leftarrow$3Q0 | I | 0.41 | 0.41 | 0.087 | 1.91 | |||
| 279.71 | 1Q1$\leftarrow$3Q0 | I | 0.088 | 1.92 | 42 | |||
| 281.73 | 3Q0 | I* | 0.029 | 1.92 | 42 | |||
| 295.91 | 3Q0 | I* | 0.030 | 1.92 | 42 | |||
| 298.23 | 1Q1$\leftarrow$3Q0 | I | 0.065 | 1.70 | 42 | |||
| 304 | 3Q0 | I* | 0.05 | 0.05 | 0.029 | 1.88 | 42 | |
| 1Q1$\leftarrow$3Q0 | I | 0.74 | 0.94 | 0.062 | 1.35 | |||
| 3Q1 | I | 0.21 | 0.057 |
2.2.2 全氟代碘甲烷(CF3I)的光解离研究
表2 CF3I光解的动力学参数Table 2 The dynamical parameters for the photodissociation of CF3I |
| λ(nm) | Pathway | Channel | Fraction | Φ(I*) | Pcc | Eint/Eavl | β | Ref. |
|---|---|---|---|---|---|---|---|---|
| 238 | 3Q0 | I* | 0.664 | 0.738 | 0.266 | 1.70 | 54 | |
| 3Q0$\leftarrow$1Q1 | I* | 0.074 | 0.294 | |||||
| 1Q1 | I | 0.178 | -0.04 | |||||
| 1Q1$\leftarrow$3Q0 | I | 0.084 | 0.112 | |||||
| 248 | 3Q0 | I* | 0.220 | 1.85 | 53 | |||
| 266 | 3Q0 | I* | 0.90 | 0.90 | 0.145 | 1.86 | 53 | |
| 1Q1$\leftarrow$3Q0 | I | 0.07 | 0.07 | 0.202 | 1.03 | |||
| 3Q1 | I | 0.03 | ||||||
| 277 | 3Q0 | I* | 0.87 | 0.87 | 0.108 | 1.86 | 53 | |
| 1Q1$\leftarrow$3Q0 | I | 0.086 | 0.09 | 0.154 | 0.98 | |||
| 3Q1 | I | 0.044 | ||||||
| 281.73 | 3Q0 | I* | 0.21 | 51 | ||||
| 304 | 3Q0 | I* | 0.06 | 0.06 | 0.12 | 1.69 | 52, 57 | |
| 1Q1$\leftarrow$3Q0 | I | 0.15 | 0.71 | 0.18 | -0.45 | |||
| 3Q1 | I | 0.79 | 0.15 |
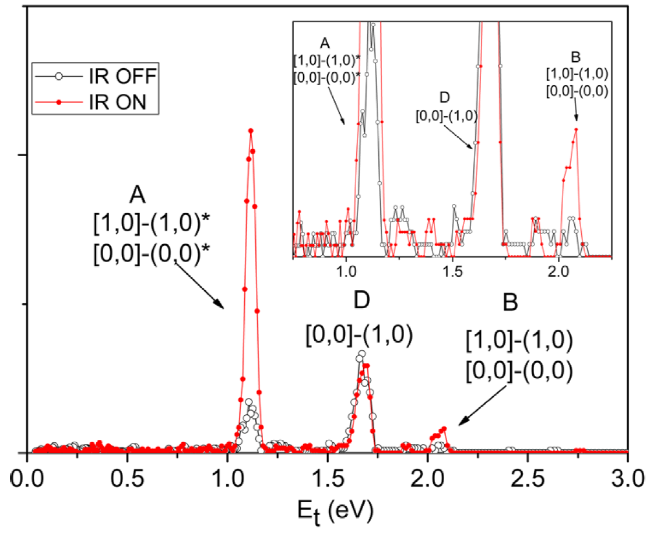
2.2.3 氯碘甲烷(I-CH2Cl)的光解离研究
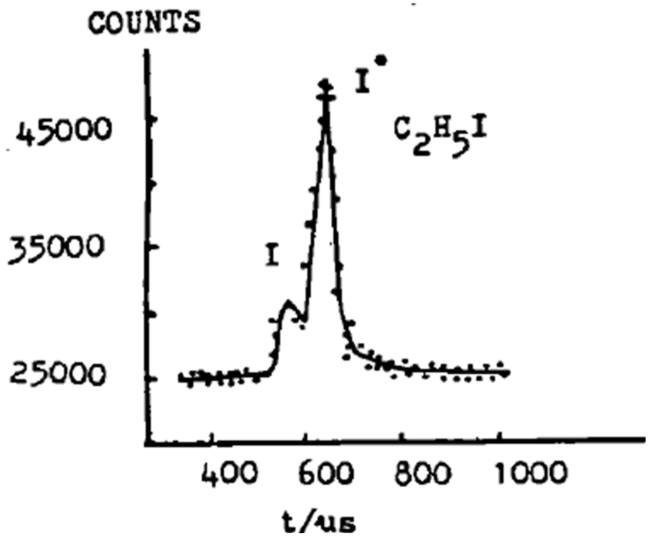
2.3 卤代乙烷的光解离研究
表3 248 nm下部分碘代烃光解离的动力学参数Table 3 The dynamical parameters for the photodissociation of partial iodohydrocarbon at 248 nm |
| Reaction Channels | Φ(I*) | Eint(R)/Eavl | Ref. |
|---|---|---|---|
| C2H5I→C2H5 + I* | 0.70 | 0.32 | 38 |
| C2H5 + I | 0.39 | ||
| n-C3H7I→n-C3H7 + I* | 0.62 | 0.49 | 38 |
| n-C3H7 + I | 0.54 | ||
| i-C3H7I→i-C3H7 + I* | 0.49 | 0.63 | 38 |
| i-C3H7 + I | 0.64 | ||
| n-C4H9I→n-C4H9 + I* | 0.31 | 0.70 | 39 |
| n-C4H9 + I | 0.74 | ||
| t-C4H9I→t-C4H9 + I | 0.76 | 39 | |
| n-C5H11I→n-C5H11 + I* | 0.67 | 0.77 | 59 |
| n-C5H11 + I | 0.76 | ||
| CH2=CHI→CH2=CH + I* | 0.57 | 0.31 | 60 |
| CH2=CH + I | 0.41 |
表4 279’305 nm范围部分碘代烃光解的动力学参数Table 4 The dynamical parameters for the photodissociation of partial iodohydrocarbon at 279~305 nm |
| λ/nm | Channels | Eint/Eavl | β | Ref |
|---|---|---|---|---|
| 281.73 | C2H5 + I* | 0.221 | - | 61 |
| 304.02 | 0.224 | - | ||
| 279.71 | C2H5 + I | 0.252 | - | 61 |
| 304.67 | 0.259 | - | ||
| 281.73 | C2F5 + I* | 0.52 | 1.70 | 16 |
| 304.02 | 0.50 | 1.64 | ||
| 279.71 | C2F5 + I | 0.60 | 1.25 | 16 |
| 304.67 | 0.55 | 0.88 | ||
| 281.73 | n-C3H7 + I* | 0.48 | 1.68 | 62 |
| 304.02 | 0.49 | ’2.00 | ||
| 279.71 | n-C3H7 + I | 0.52 | ’2.00 | 62 |
| 304.67 | 0.52 | 1.57 | ||
| 281.73 304.02 | i-C3H7 + I* | 0.61 | 1.72 | 62 |
| 0.65 | 1.75 | |||
| 279.71 | i-C3H7 + I | 0.62 | 1.32 | 62 |
| 304.67 | 0.49 | 1.31 |
2.4 其他卤代烃的光解离研究
3 重要小分子的光电离动力学研究
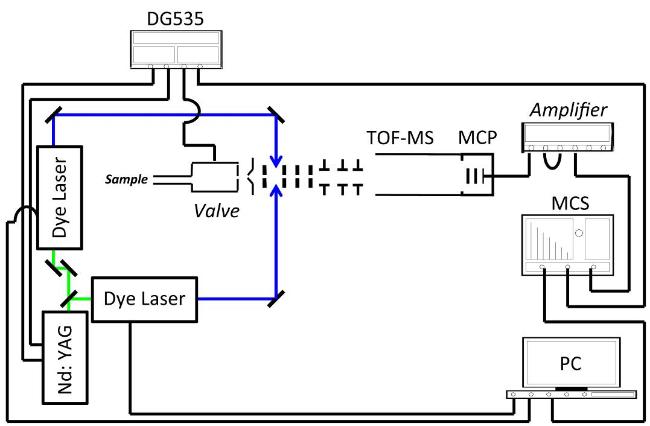
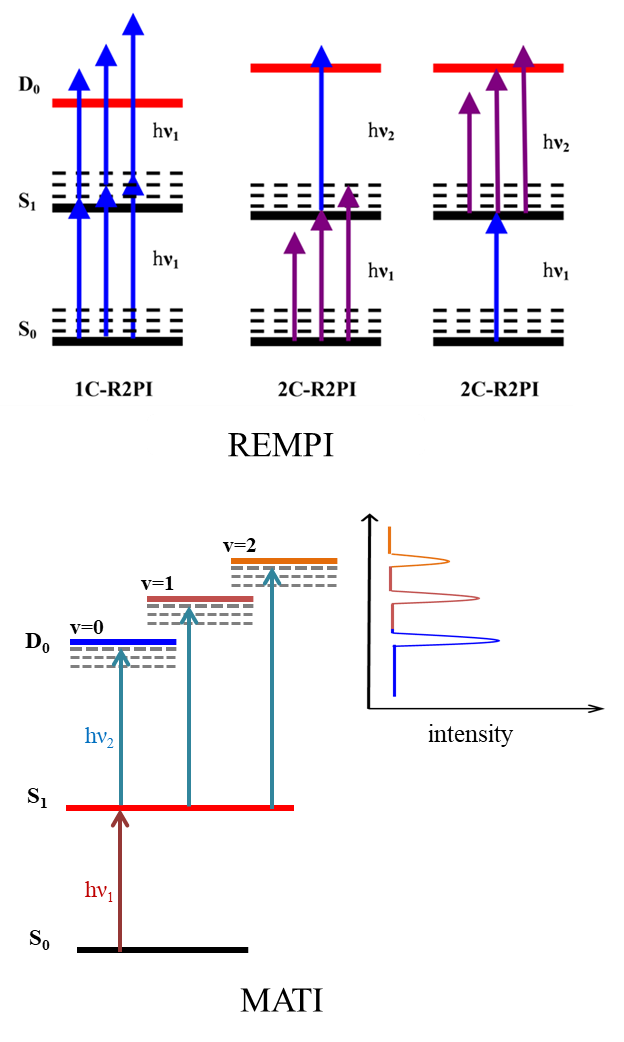
3.1 REMPI/MATI和IR-UV光谱实验装置
图13 REMPI/MATI光谱实验装置示意图Fig. 13 The schematic diagram of REMPI/MATI spectroscopy experimental device |
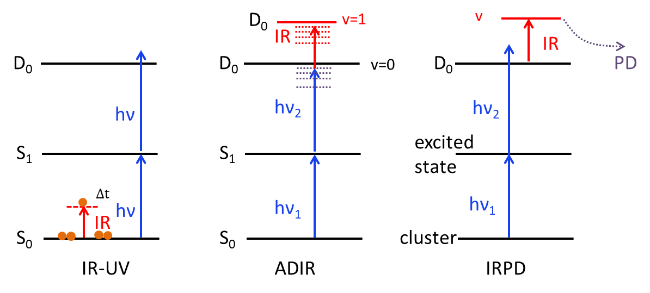
图15 IR-UV、ADIR和IRPD光谱原理示意图Fig. 15 The principles of IR-UV, ADIR, and IRPD spectroscopy |
表5 若干典型苯衍生物分子的第一电子激发态跃迁能(E1)和电离能(IE)汇总表Table 5 Summary of electronic transition energies (E1) and ionization energies (IE) for several typical benzene derivative molecules |
| Molecules | E1 (cm−1) | IE (cm−1) | Ref. |
|---|---|---|---|
| cis m-fluorostyrene | 34403 | - | 65 |
| trans m-fluorostyrene | 34663 | - | 65 |
| p-methylstyrene | 34276 | - | 66 |
| p-fluoroanisole | 35149 | - | 67 |
| cis p-methoxystyrene | 33242 | - | 68 |
| trans p-methoxystyrene | 33324 | - | 68 |
| p-chloroanisole | 34859 | - | 69 |
| cis 3-chloro-4-fluoroanisole | 34703 | 67349 | 70 |
| trans 3-chloro-4-fluoroanisole | 34747 | 67595 | 70 |
| cis 3-chlorostyrene | 33766 | 69701 | 71 |
| trans 3-chlorostyrene | 34061 | 69571 | 71 |
| cis m-aminostyrene | 30937 | 61278 | 72 |
| trans m-aminostyrene | 31140 | 61495 | 72 |
| 3,5-difluoroanisole | 37595 | 70096 | 73 |
| cis 3-chloro-5-fluoroanisole | 36468 | 69720 | 74 |
| trans 3-chloro-5-fluoroanisole | 36351 | 69636 | 74 |
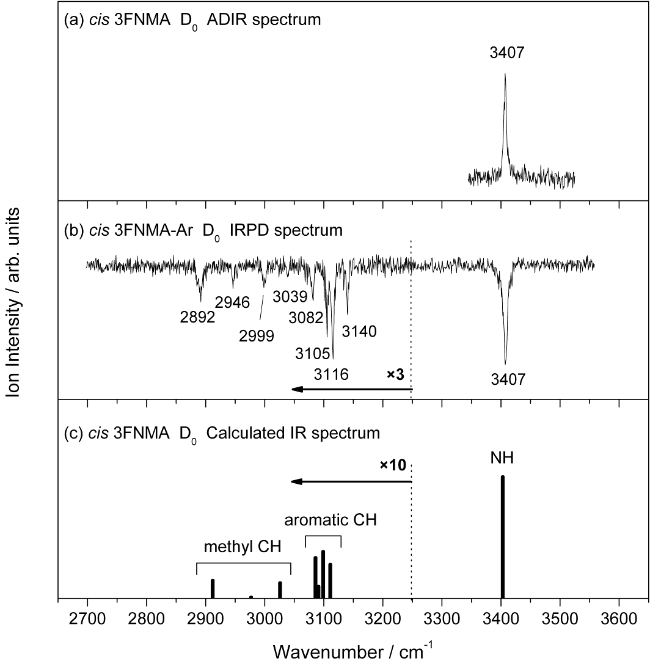
| cis 3-fluoro-N-methylaniline | 33816 | 61742 | 75, 76 |
| trans 3-fluoro-N-methylaniline | 34023 | 61602 | 75, 76 |
| cis 4-chloro-3-fluoroanisole | 35443 | 67585 | 77 |
| trans 4-chloro-3-fluoroanisole | 35326 | 67324 | 77 |
| trans 2-fluoro-N-methylaniline | 34010 | 61101 | 78 |
| cis 3-chloro-N-methylaniline | 33003 | 61531 | 79 |
| trans 3-chloro-N-methylaniline | 32886 | 61625 | 79 |
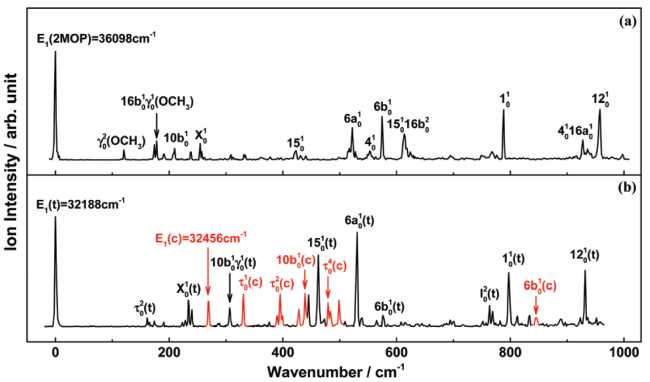
| cis 2-methoxypyridine | 36098 | 69379 | 80 |
| cis 2-N-methylaminopyridine | 32456 | 62518 | 80 |
| trans 2-N-methylaminopyridine | 32188 | 62709 | 80 |