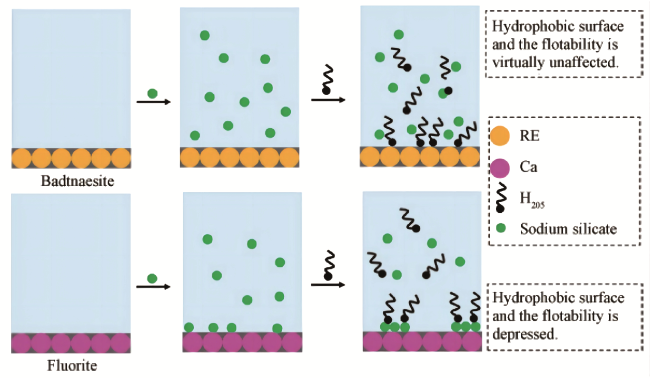
1 引言
2 稀土浮选捕收剂
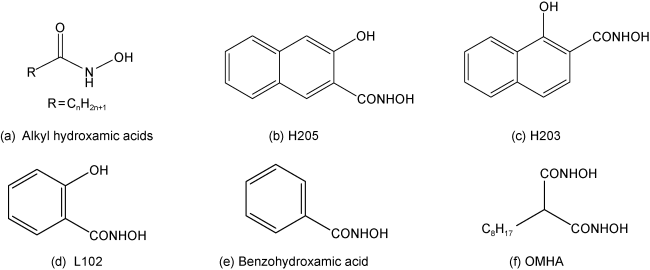
2.1 羟肟酸类捕收剂
表1 部分羟肟酸捕收剂的稀土浮选性能研究Table 1 Study of rare earth flotation performance of some hydroxamic acids |
| Collector | Raw material | Flotation reagents | Concentrate |
|---|---|---|---|
| C7-C9 hydroxamic acid[19] | Rare earth ore from Australia with 19.13% rare earth oxide grade (REO). It mainly contains bastnaesite, monazite, limonite, magnetite and hematite. | Primary flotation: C7-C9 hydroxamic acid 1 kg/t, NaOH 1 kg/t, water glass 6.4 kg/t, humic acid 0.2 kg/t, sodium fluosilicate 1 kg/t, NaS 0.7 kg/t. | After a rough flotation, rare earth concentrate with 30.30% REO and 32.46% recovery was obtained. |
| H203、H205[20] | Rare earth ore from Bao steel processing plant (China) with 11.20% REO. It mainly contains bastnaesite, monazite, limonite, and silicate minerals. | Primary flotation: H203 or H205 4.0 kg/t, water glass 4.0 kg/t, XJ-01 foaming agent 0.048 kg/t, temperature 36~38 ℃. | After a rough flotation, H203 yields a rare earth concentrate with 31.54% REO and 79.05% recovery; H205 yields a rare earth concentrate with 36.77% REO and 79.40% recovery. |
| L102[21] | Rare earth ore from Bao steel processing plant (China) with 22.80% REO. It mainly contains bastnaesite, monazite, Iron minerals, fluorite, sodium pyroxene, sodium amphibole, and dolomite. | Primary flotation: Salicylic acid 2.85 kg/t, water glass 3.30 kg/t, 2# oil 0.076 kg/t, pulp concentration 45~50%, pH=8.5~9.5, temperature 40 ℃. | After "one rough and two fine" closed circuit flotation, a rare earth concentrate with 63.50% REO and 56.32% recovery was obtained. |
| H205、 H316[15] | Rare earth ore from Bao steel processing plant (China) with 10.10% REO. It mainly contains bastnaesite, monazite, Fluorite, apatite, hematite, pyrite, feldspar, quartz, mica, etc. | Bao steel ore dressing plant dilution workshop "one rough two fine" closed-circuit industrial flotation, the daily capacity of 465 t, the colectort was H316, sodium silicate as inhibitors, and H103 used as foaming agent. | After "one rough and two fine" flotation, H205 yields a rare earth concentrate with 53.09% REO and 67.11% recovery; H316 yields a rare earth concentrate with 53.31%; REO and 72.98% recovery. |
| P8[22] | Rare earth ore from Baotou (China) with 9.60% REO. It mainly contains fluorite, hematite, bastnaesite, sodium pyroxene, sodium amphibole, monazite and barite. | Primary flotation: P8 (the main component is H205) 2.4 kg/t; water glass 5.6 kg/t; turpentine oil 0.36 kg/t; temperature 65 ℃, concentration 30 wt%, pH=8~9. | After "one rough, three fine and one sweep" flotation, a rare earth concentrate with 50.3% REO and 78.6% recovery was obtained. |
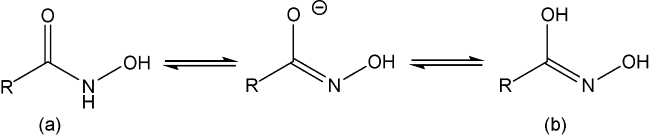
2.1.1 羟肟酸的合成
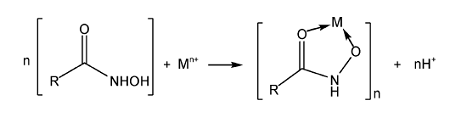
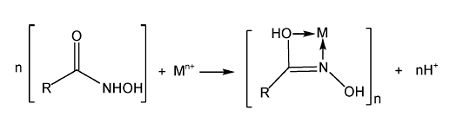
2.1.2 羟肟酸捕收机理
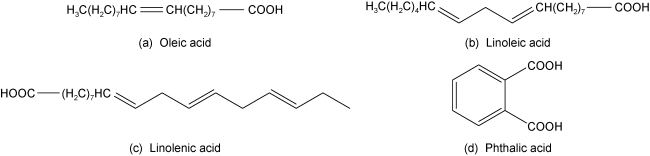
2.2 脂肪酸类捕收剂
图3 部分脂肪酸捕收剂主要成分结构Fig. 3 Structure of the main components of some fatty acid collectors |
表2 部分脂肪酸类捕收剂浮选性能研究Table 2 Study of rare earth flotation performance of some fatty acid collectors |
| Collector | Raw material | Flotation reagents | Concentrate |
|---|---|---|---|
| sodium oleate [50] | Rare earth ore from an Australian processing plant with 1.07% REO. It mainly contains bastnaesite, monazite, quartz, mica, micas, magnetite and hematite. | Primary flotation: Sodium oleate 3.0 kg/t, water glass 1.5 kg/t, starch 1.5 kg/t. | After "one rough, one sweep and one concentrate" flotation, a rare earth concentrate with REO 2.25% and 63% recovery was obtained. |
| sodium oleate [51] | Rare earth ore from a processing plant in Bayan Obo (China) with 9.35% REO and CaF2 grade 26.49%. It mainly contains bastnaesite, monazite, Fluorite, hematite, magnetite, pyroxene, amphibole, quartz and feldspar. | Flotation of rare earths: SR (mixture of hydroxamic acid and Sodium Carbonate) as the collector. Primary flotation of fluorite: Sodium oleate 0.6 kg/t, acidic water glass 1.4 kg/t, SY (mixture of sodium hexametaphosphate and tannic acid) 1.28 kg/t. | After “rare earth flotation - fluorite pre-selection - fluorite selection - strong magnetic separation”, it can obtain rare earth concentrate with 50.54% REO and 92.32% recovery; and fluorite concentrate with 95.51% CaF2 grade and 50.98% recovery. |
| Tarr Oil [42] | Rare earth ore from Muntin Pass Rare Earth Mine (USA) with 7.7% REO. It mainly contains bastnaesite, Calcite, barite, quartz, etc. | Flotation process at the rare earth ore dressing plant in Muntin Pass, USA, with Tar oil as a collector and lignosulfonic acid as a depressant. | Rare earth concentrate with REO 65% and 80% recovery was obtained. |
| phthalic acid [52] | Fluorite concentrate from a processing plant in Bayan Obo (China) with a grade of 86.02% CaF2 and 5.72% REO. t mainly contains fluorite, bastnaesite, Calcite, ilmenite, quartz, etc. | Primary flotation: Phthalic acid 2.4 kg/t, water glass 2 kg/t, pH=5. | After "one rough and three fine" closed-circuit flotation, the fluorite concentrate with a grade of 95.12% CaF2 and a recovery of 84.05% was obtained. |
| phthalic acid [53] | Baotou mixed rare earth concentrates: bastnaesite (REO 51.18%, purity 79.99%), monazite (REO 12.80%, purity 20.01%), and it also contains fluorite and barite. | Primary flotation: Phthalic acid 2.2 kg/t, alum as depressant 4.0 kg/t. | After "one rough and two sweeps" bastnaesite concentrate (REO 68.81%, purity 95.04%) and monazite concentrate (REO 58.55%, purity 95.34%) were obtained. |
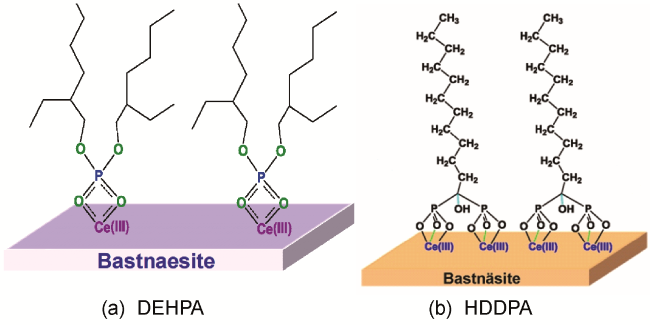
2.3 磷酸类捕收剂
表3 部分磷酸类捕收剂浮选性能研究Table 3 Study of rare earth flotation performance of some phosphonic acid collectors |
| Collector | Raw material | Flotation reagents | Concentrate |
|---|---|---|---|
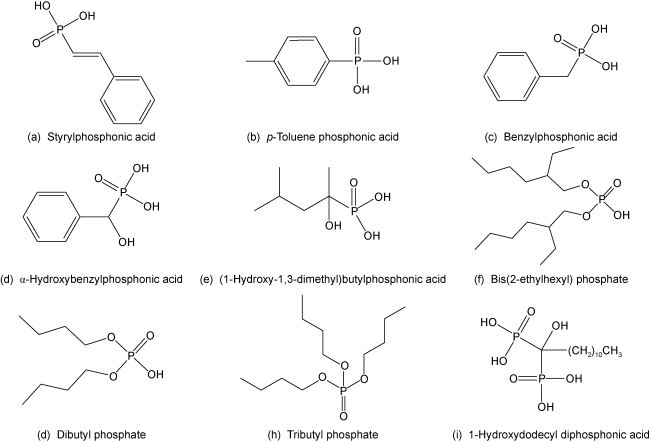
| Styrylphosphonic acid [60] | Shandong Huishan (China) rare earth ore with 6.10% REO. It mainly contains bastnaesite, Parisite, hematite, limonite. | Primary flotation: Sulfuric acid 0.5 kg/t, kerosene 2.5 kg/t, Styrophylphosphonic acid 1.5 kg/t, pine oil 0.2 kg/t. | After "One rough and two fine" open circuit flotation, a rare earth concentrate with 60.13% REO and 48.36% recovery was obtained. |
| Styrene phosphonic acid, toluene phosphonic acid, alpha-hydroxybenzylphosphonic acid, benzylphosphonic acid, (1-hydroxy-1,3- dimethyl) butylphosphonic acid[46] | the same as above | Different collectors use different optimal flotation conditions. | Rare earth concentrates were obtained: styrylphosphonic acid (REO 41.67%, recovery 50.50%); toluene phosphonic acid (REO 43.23%, recovery 37.29%); α-hydroxybenzylphosphonic acid (REO 55.55%, recovery 66.35%); benzylphosp- honic acid (REO 34.39%, recovery 44.05%); and (1-hydroxy -1,3- dimethyl) butylphosphonic acid (REO 33.77%, recovery 53.69%). |
| P538[61] | Baotou mixed rare earth concentrates: REO > 68%, bastnaesite 71.67%, Monazite 28.33%. | Multi-stage flotation, P538 as collector added in batches, rough flotation reagent was: citric acid 200 mg/L, frothing agent MIBC 12 mg/L, pH=5.0. | Bastnaesite concentrate with purity of 91% and recovery of 70.18% and monazite concentrate with purity of 61.2% and recovery of 73.18% were obtained. |
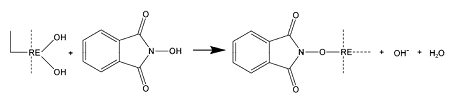
2.4 其他捕收剂
3 起泡剂
4 调整剂
4.1 抑制剂
表4 其他报导的部分稀土浮选抑制剂Table 4 Other reported flotation depressants of rare earth |
| depressant | Inhibited Mineral | Possible Mechanisms of Inhibition | disadvantages | |
|---|---|---|---|---|
| Alum [86,87] | It has good inhibition effect on barite, fluorite, calcite and other barium calcium salt minerals. And it can also be used to inhibit monazite when separating bastnaesite and monazite. | Alum hydrolyzed Al3+ preferentially combines with SO42- on the surface of barite to inhibit barite. Ca2+ on the surface of calcite and fluorite preferentially combines with AlO2- and SO42- hydrolyzed by alum to inhibit calcite and fluorite. | Bastnaesite will be partially inhibited when alum used in excess. | |
| Lignosulfonate [86,87] | It can effectively inhibit barite and calcite, and there is little difference in the inhibition effect under high temperature conditions. | It contains phenolic hydroxyl functional groups, can be selectively adsorbed on the surface of barite and calcite to make the mineral surface hydrophilic. | Needs to control the dosage, overuse is not conducive to the flotation of rare earth ores, such as bastnaesite. | |
| Sodium fluorosilicate [86] | It can be used to inhibit barite, calcite, fluorite, quartz, feldspar, and other silicates. | Hydrolyzed SiF62- continues to be hydrolyzed to SiO2 micelles adsorbed on the mineral surface making the mineral hydrophilic. Hydrolyzed SiF62-continues to be hydrolyzed to HF, which dissolves the dissolved feldspar surface to generate free H2SiO3 micelles, preventing the capture of the trapper. It can preferentially desorb fatty acid-based traps from the surface of vein minerals. | It results in a lower pH of the slurry, requiring the addition of more pH adjusters such as sodium carbonate. | |
| Sodium hexametaphosphate [54,57,88] | It can be used to inhibit calcite and barite. | Hydrolyzed Na4P6O182- forms hydrophilic and stable complexes with Ca2+ on the mineral surface in the slurry. | Has a strong inhibition effect on Bastnaesite, so it needs to strictly control the dosage | |
| Sodium sulfide [89] | It can be act as inhibitors of zircon and activators of monazite when fatty acid as collector | Hydrolyzed S2- and HS- ions are adsorbed on the surface of zircon, preventing the adsorption of collectors on zircon or leading to selective resolution of collectors such as oleic acid on the surface of zircon. | Easily releases toxic hydrogen sulfide gas in the air. | |
| Citrate [61] | It can inhibit fluorite and aluminum silicate minerals such as calcite and mica. And it can also be used to inhibit bastnaesite when separating monazite and bastnaesite in flotation. | Citric acid and mineral lattice surface cations have a strong ability to cooperate with the adsorption on the surface of the inhibited minerals, and forming hydrophilic chelates to hinder the adsorption of the collectors on the gangue minerals. Citric acid has a greater ability to dissolve cerium fluorocarbon, and the mineral surface loses more active centers and is subsequently inhibited. | High acidity will reduce the pH of the slurry, and dosage also needs to be controlled. | |
| Starch, modified starch [90⇓-92] | It can be used to inhibit rutile, ilmenite, zircon, hematite, quartz and so on. | Polar functional groups such as hydroxyl groups on the molecular chain are adsorbed on the mineral surface through hydrogen bonding, electrostatic and chelating effects, and are inhibited by the formation of a hydrophilic adsorption layer on the mineral surface. | Natural starch has poor water solubility and poor selective adsorption capacity on mineral surfaces. Modified starch has small applicability, and mostly stays in the laboratory research stage. | |
| EDTA[93⇓-95] | It is mainly used to inhibit calcium-containing minerals such as fluorite and calcite. Low doses can remove Ca2+ ions from the surface of monazite and realize the activation of monazite, but it also has a certain inhibition effect on monazite in large doses. | Complexation with dissolved Ca2+ on the mineral surface hinders the adsorption of collectors such as hydroxamic acid on the surface of minerals such as fluorite. | Dosage needs to be controlled and often requires synergistic use with other inhibitors. | |
| CMC[86,96] | It can be used to inhibit silicate minerals and minerals containing calcium and magnesium. | After hydrolysis, carboxymethyl anions are electrostatically attracted to the cations on the mineral surface, carboxyl groups form a water film with water through hydrogen bonding, and part of the CMC is adsorbed on the mineral surface as negatively charged micelles, which ultimately results in the hydrophilicity of the mineral surface and its inhibition. | Requires synergistic use with other inhibitors. | |
| Guar gum [97] | Inhibits minerals such as talc and silicates | Similar to starch and modified starch inhibition mechanism | It is easy to cause the slurry to become viscous, reduce the flotation grade, so it needs to strictly control the dosage. | |
4.2 活化剂
5 离子型稀土矿化学选矿试剂
5.1 浸出剂
表5 部分试剂浸出某离子型稀土矿试验结果[106]Table 5 Leaching results of some leaching reagents on an ion-adsorption type rare earths ores[106] |
| Leaching agent | Concentration | pH | Leaching rate of RE /% | Leaching agent | Concentration | pH | Leaching rate of RE /% |
|---|---|---|---|---|---|---|---|
| HCl | 2% | 0.5 | 52.92 | KCl | 1 mol/L | 5.4 | 92.99 |
| H2SO4 | 2% | 0.5 | 76.09 | Ammonium citrate | 0.5 mol/L | 4.5 | 95.18 |
| NH4Cl | 1 mol/L | 5 | 94.72 | Fe2(SO4)3 | 1% | 2.5 | 70.00 |
| CH3COONH4 | 1 mol/L | 6 | 94.66 | FeSO4 | 1% | 2.5 | 67.00 |
| NaCl | 1 mol/L | 5.4 | 97.53 | (NH4)2SO4 | 2% | 4.5 | 98.50 |