1 引言
2 基于NMOFs的单一治疗
2.1 CT
图1 构建MOFs纳米系统进行CT。(A)DOX@ZIF-8@eM-cRGD的制备及其用于肿瘤CT的示意图[33];(B)FZIF-8/DOX-MIPs的合成和降解示意图[34];(C)A-RAMP的制备示意图[35]Fig. 1 The MOFs nanosystems was constructed for CT. (A) Schematic diagram of DOX@ZIF-8@eM-cRGD preparation and application in tumor therapy[33]; Copyright 2020, American Chemical Society. (B) Schematic diagram of FZIF-8/DOX-MIPs preparation and degradation[34]; Copyright 2020, American Chemical Society. (C) Schematic diagram of A-RAMP preparation[35]; Copyright 2020, American Chemical Society |
2.2 PTT
图2 NMOFs在肿瘤PTT中的应用。(A)Mn-IR825@PDA-PEG的制备示意图[39];(B)MIL-100(Fe)@HA@ICG的制备示意图[44];(C)Au@ZIF-8的制备和金纳米球释放及自组装的示意图[45]Fig. 2 Application of NMOFs in tumor PTT. (A) Schematic diagram of Mn-IR825@PDA-PEG preparation[39]; Copyright 2016, American Chemical Society. (B) Schematic diagram of MIL-100(Fe)@HA@ICG preparation[44]; Copyright 2017, American Chemical Society. (C) Schematic diagram of Au@ZIF-8 preparation and Au nanosphere release and self-assembly[45]. Copyright 2020. American Chemical Society |
2.3 PDT
图3 NMOFs在肿瘤PDT中的应用。(A)PCN-224的制备和单线态氧产生示意图[52];(B)mCGP的制备示意图[55];(C)PCN-224-Pt的制备和增强PDT示意图[56]Fig. 3 Application of NMOFs in tumor PDT. (A)Schematic diagram of PCN-224 preparation and singlet oxygen generation[52]; Copyright 2016, American Chemical Society. (B) Schematic diagram of mCGP preparation[55]; Copyright 2017, American Chemical Society. (C)Schematic diagram of PCN-224-Pt preparation and enhanced PDT[56]. Copyright 2018, American Chemical Society |
2.4 CDT
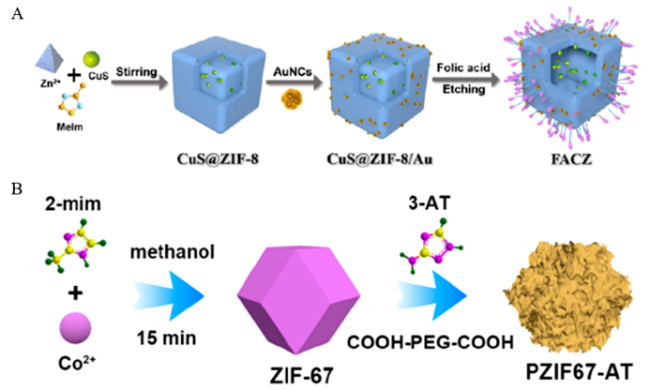
图4 NMOFs在肿瘤CDT中的应用。(A)ZIF-8/Au/CuS/FA[66]和(B)PZIF67-AT的制备示意图[70]Fig. 4 Application of NMOFs in tumor PDT. (A) Schematic diagram of ZIF-8/Au/CuS/FA preparation[66]; Copyright 2020, American Chemical Society. (B) Schematic diagram of PZIF67-AT preparation[70]. Copyright 2020, American Chemical Society |
3 基于NMOFs的联合治疗
3.1 双模式联合治疗
3.1.1 CT和PTT联合治疗
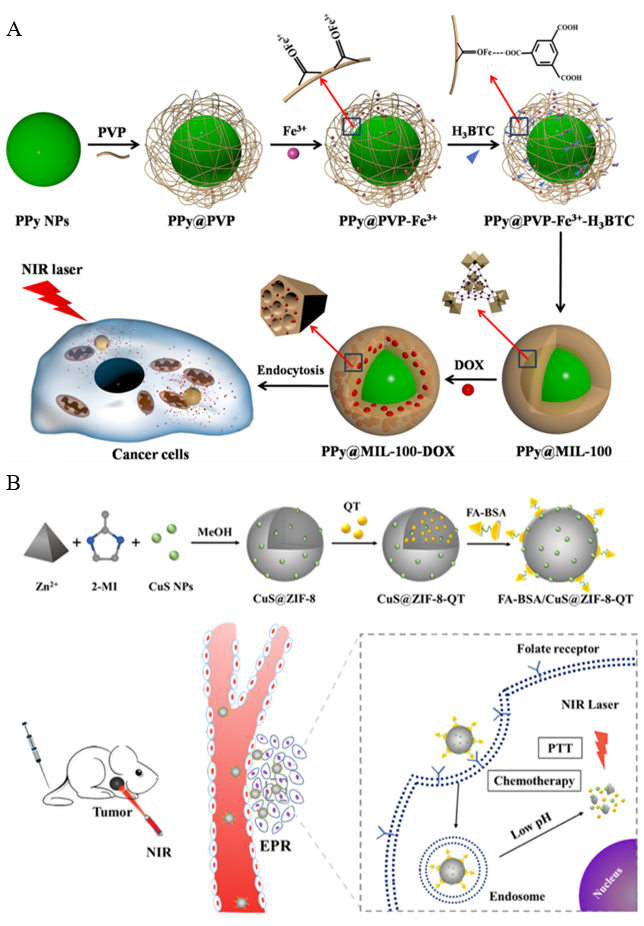
图5 NMOFs在肿瘤CT-PTT中的应用。(A)PPy@MIL-100-DOX复合纳米材料示意图[72];(B)FA-BSA/CuS@ZIF-8-QT的制备和体内治疗示意图[75]Fig. 5 Application of NMOFs in tumor CT-PTT. (A) Schematic diagram of PPy@MIL-100-DOX composite nanomaterials[72]; Copyright 2016, American Chemical Society. (B) Schematic diagram of FA-BSA/CuS@ZIF-8-QT preparation and in vivo therapy[75]. Copyright 2018, American Chemical Society |
3.1.2 CT和PDT联合治疗
3.1.3 PTT和PDT联合治疗
3.1.4 CT和CDT联合治疗
3.1.5 PDT和CDT联合治疗
3.1.6 PTT和CDT联合治疗
3.2 三模式联合治疗
3.2.1 PTT-PDT-CT
3.2.2 PTT-PDT-CDT
4 总结与展望
表1 基于NMOFs材料的肿瘤治疗应用总结Table 1 Summary of the application of tumor therapy based on NMOFs material |
| Drug delivery based on NMOFs | NMOFs components | Shape | Size (nm) | Cargos | Loading capacity | Modification | Targeting mechanism | Response types | Wavelengths (nm) | Cell line | Functionality | ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DOX/Fe(bbi)@SiO2-FA | Fe2+, bbi | 3D | 200 | DOX | 98% | SiO2 | FA | pH | - | HeLa | CT | 23 | |
| PEG-FA/PEGCG@ZIF-8 | Zn2+, 2-mim | 3D | 220 | PEGCG | 19.9% | PEG | FA | pH | - | HEK 293/HeLa | CT | 31 | |
| PEG-FA/(DOX+VER)@ZIF-8 | Zn2+, 2-mim | 3D | 180 | DOX+VER | 40.9% | PEG | FA | pH | - | B16F10/MCF-7A | CT | 7 | |
| CAD@ZIF-8-FA | Zn2+, 2-mim | 3D | 150 | DOX | 34.75% | - | FA | pH | - | MDA-MB-231/MCF-10A | CT | 32 | |
| DOX@ZIF-8@eM-cRGD | Zn2+, 2-mim | 3D | 110 | DOX | 49% | PEG | cRGD | pH | - | HeLa/MDA-MB-231/ RAW264.7 | CT | 33 | |
| GEM⊂RGD@nZIF-8 | Zn2+, 2-mim | 3D | 98 | GEM | 7.8% | - | RGD | pH | - | A549 | CT | 96 | |
| FZIF-8/DOX-MIP | Zn2+, 2-mim | 3D | 172 | DOX | - | - | MIP | GSH/pH | - | 293T/LoVo/MCF-7 | CT | 34 | |
| DSF@HA/Cu-MOF | Cu2+, 2-MI | 3D | 313 | DSF | 54.17% | - | HA | pH | - | 4T1/ L02 | CT | 97 | |
| DOX@ZIF-8@P(MPC-co-C7A) | Zn2+, 2-mim | 3D | 285 | DOX | 7% | P(MPC-co-C7A) | P(MPC-co-C7A) | pH | - | A549 | CT | 98 | |
| DOX-HAp@Lys/ZIF-8 | Zn2+, 2-mim | 3D | 600 | DOX | 56.5% | Lys | HAp | pH | - | HeLa | CT | 99 | |
| CD20-RBCm@Ag-MOFs/ PFK15 | Ag+, 2-mim | 3D | 109 | PFK15 | 68.6% | RBCm | CD20 Apt | pH | - | Raji/K562/RAW264.7/OCI-LY8/ OCI-LY10 | CT | 35 | |
| Fe3O4@MOF-DOX-CDs-Apt | Zr4+, NH2-BDC | 3D | 26 | DOX | - | - | AS1411 | pH | - | HUVEC/MDA-MB-231 | CT | 36 | |
| Mn-IR825@PDA-PEG | Mn2+, IR825 | 3D | 40 | IR825 | - | PDA-PEG | - | - | 808 | 293T /HeLa/A549 | PTT | 39 | |
| Zr-PDI | Zr4+, PDI | 3D | - | PDI | - | - | - | - | 808 | - | PTT | 40 | |
| Fe-CPND | Fe3+,gallic acid | 3D | 5.3 | - | - | PVP | - | - | 808 | SW620 | PTT | 41 | |
| Fe-EA | Fe3+, EA | 3D | 240 | - | - | - | - | - | 808 | 4T1 | PTT | 43 | |
| MIL-100(Fe)@HA@ICG | Fe3+, H3BTC | 3D | 100 | ICG | 42% | - | HA | - | 808 | MCF-7 | PTT | 44 | |
| Au@ZIF-8 | Zn2+, 2-mim | 3D | 115 | AuNPs | - | - | - | GSH/pH | 808 | 4T1/HUVEC | PTT | 45 | |
| Hf-DBP | Hf4+, H2DBP | 3D | 100 | H2DBP | - | - | - | - | 640 | SQ20B | PDT | 51 | |
| PCN-224 | Zr4+, TCPP | 3D | 90 | TCPP | - | - | FA | - | 660 | HeLa/A549 | PDT | 52 | |
| PS@MOF | - | 3D | 40 | TMPyP | 32.8% | PEG | FA | - | 660 | HeLa | PDT | 53 | |
| PS@MOF-199 | Cu2+, H3BTC | 3D | 120 | Ce6 | 49% | F-127 | - | GSH | 400~700 | HepG2/NIH-3T3 | PDT | 54 | |
| PMOF-199 | Cu2+, H3BTC | 3D | 80 | TPATrzPy3+ | 51.3% | F-127 | - | GSH | 400~700 | HeLa/3T3 | PDT | 100 | |
| PS@ZIF-8-PMMA-S-S-mPEG. | Zn2+, 2-mim | 3D | 50 | D-A PS | - | PEG | - | GSH | 400~700 | 4T1 | PDT | 101 | |
| ZnP@Hf-QC | Hf4+, H2QC | 3D | 167 | ZnP | 36.1% | - | - | - | 700 | CT26 | PDT | 57 | |
| Ce6/Cytc@ZIF-8/HA | Zn2+, 2-mim | 3D | 128 | Ce6 | 7.15% | - | HA | pH | 670 | L929/HeLa | PDT | 58 | |
| PCN-58-Ps-HA | Zr4+,TPDC- 2CH2N3 | 3D | 160 | Ps | 83.3% | - | HA | - | 910 | HEK 293T/ HeLa | PDT | 102 | |
| mem@Catalase@GOx@PCN-224 | Zr4+, TCPP | 3D | 227 | Gox/CAT | 13.5% | mem | mem | - | 66 | 4T1/COS7/RAW264.7 | PDT | 55 | |
| Drug delivery based on NMOFs | NMOFs components | Shape | Size (nm) | Cargos | Loading capacity | Modification | Targeting mechanism | Response types | Wavelengths (nm) | Cell line | Functionality | ref | |
| mem@MnO2@MOF | Zr4+, TCPP | 3D | 105 | TCPP | - | mem | mem | - | 409 | HeLa/ HepG2/3T3 | PDT | 103 | |
| PCN-224-Pt | Zr4+, TCPP | 3D | 92 | TCPP | - | PEG | - | - | 660 | HeLa/RAW264.7/4T1 | PDT | 56 | |
| MnFe2O4/C@Ce6 | Mn2+, Fe3+, fumaric acid | 3D | 160 | Ce6 | 11.3% | - | - | - | 660 | U-87 MG | PDT | 60 | |
| MON-p53 | Fe3+, tannic acid | 3D | 60 | Fe2+ | - | - | - | - | - | HT1080/SCC-7/4T1/COS-7/ MCF7 | CDT | 65 | |
| ZIF-8/Au/CuS/FA | Zn2+, 2-mim | 3D | 279 | CuS | - | - | FA | pH | - | HCMEC/D3/HepG-2 | CDT | 66 | |
| GOx@Pd@ZIF-8 | Zn2+, 2-mim | 3D | 130 | Pd nano-enzyme | 3.59% | - | - | pH | - | A549 | CDT | 68 | |
| Fe@ZIF-8@GOx | Zn2+, 2-mim | 3D | 635 | Fe nano-enzyme | - | - | - | pH | - | HeLa | CDT | 69 | |
| PZIF67-AT | Co2+, 2-mim | 3D | 180 | 3-AT | 28.5% | PEG | - | pH | - | HeLa/A549/4T1 | CDT | 70 | |
| DOX@Fe-CNPs | Fe3+, HCA | 3D | 138 | DOX | 14.5% | - | - | pH/NIR | 808 | 4T1 | CT-PTT | 42 | |
| PPy@MIL-100(Fe)/DOX | Fe3+, H3BTC | 3D | 107 | PPy/DOX | 12.8% | - | - | pH/NIR | 808 | Hela | CT-PTT | 72 | |
| MCH | Fe3+, H3BTC | 3D | 200 | curcumin /PDA | 94.3% | PDA | HA | pH | 808 | HeLa/A549/CHO/MRC-5 | CT-PTT | 24 | |
| Au@Cu3(BTC)2@Apt-DOX | Cu2+, H3BTC | 3D | - | DOX/AuNPs | 57% | - | Apt | pH | 808 | A549/beas-2b/MCF-7/HeLa | CT-PTT | 73 | |
| AuNR@ZIF-8-DOX | Zn2+, 2-mim | 3D | 150 | DOX/ AuNPs | 35.8% | - | - | pH/NIR | 808 | 4T1 | CT-PTT | 104 | |
| DOX/Pd@Au@ZIF-8 | Zn2+, 2-mim | 3D | 254 | DOX/ AuNPs | 3.93% | - | - | pH/NIR | 780 | SMMC-7721 | CT-PTT | 105 | |
| CSD-MOFs@DOX | Zn2+, 2-mim | 3D | 120 | DOX/PB | 85.23% | - | - | pH/NIR | 808 | HeLa | CT-PTT | 74 | |
| Gd/Tm-PB@ZIF-8/PDA-DOX | Zn2+, 2-mim | 3D | 200~300 | DOX/ Gd/Tm-PB | 8.1% | PDA | - | pH/GSH | 808 | 4T1 | CT-PTT | 106 | |
| HmPGTL | Fe3+,K4[Fe (CN)6] | 3D | 80 | TLND | 23.5% | mem | mem | pH | 808 | L929/ HepG2 | CT-PTT | 107 | |
| FA-BSA/CuS@ZIF-8-QT | Zn2+, 2-mim | 3D | 45 | QT/ CuS | 24% | - | FA | pH | 808 | B16F10 | CT-PTT | 75 | |
| BYL719&Cisplatin@Au@MOF@MS-ICG | Zn2+, 2-mim | 3D | 124 | BYL719/Cisplatin/ICG | 32% | PAA | dYNH | pH | 808 | A549/HOB/HBMSC | CT-PTT | 108 | |
| GNRs-MSNs-MA | Fe3+, H3BTC | 3D | 160 | DOX/GNR | 23.5% | - | HA | pH/NIR | 808 | 4T1/MCF-7/HUVEC/RAW264.7 | CT-PTT | 109 | |
| DOX/Pd@ZIF-8@PDA | Zn2+, 2-mim | 3D | 110 | DOX/ PDA | 12% | PDA | - | pH/NIR | 808 | 4T1 | CT-PTT | 110 | |
| g-C3N4@ZIF-8-DOX | Zn2+, 2-mim | 3D | 60 | DOX | 35% | - | - | pH | 390~780 | A549 | CT-PDT | 77 | |
| UC@mSiO2-RB@ZIF-O2-DOX/PEG-FA | Zn2+, 2-ICA | 3D | 140 | RB/DOX | 11.6% | - | PEG-FA | pH | 808 | 4T1/HeLa | CT-PDT | 78 | |
| DOX@MnCPs/PEG | Mn3+, HMME | 3D | 100 | DOX/HMME | - | PEG | - | GSH | 630 | 4T1 | CT-PDT | 111 | |
| UCNP@MOF-DOX | Al3+, CTAB | 3D | 47~62 | DOX | 7.5% | - | - | pH | 980 | HeLa | CT-PDT | 112 | |
| HA-DOX-PCN | Zr4+, TCPP | 3D | 250 | DOX/TCPP | 108% | - | HA | pH | 640 | Hek 293T/MDA-MB- 231/SCC-7 | CT-PDT | 79 | |
| PCN@MnO2@DOX@HA | Zr4+, TCPP | 3D | 100 | DOX/TCPP | 10.3% | MnO2 | HA | pH/GSH | 638 | CT26/COS7 | CT-PDT | 80 | |
| Drug delivery based on NMOFs | NMOFs components | Shape | Size (nm) | Cargos | Loading capacity | Modification | Targeting mechanism | Response types | Wavelengths (nm) | Cell line | Functionality | ref | |
| PTFCG@MH | Fe3+, TA | 3D | 160 | Ce6/GOx | 13.3% | MnO2 | HA | pH/GSH | 635 | MDA-MB-231/ | CT-PDT | 113 | |
| DOX@Gal-PCN-224 | Zr4+, TCPP | 3D | 121 | DOX/TCPP | 14.5% | HOOC-PEG-COOH | Gal | pH | 660 | HepG2/Huh7/HEK293/L02 | CT-PDT | 81 | |
| Mn/DVDMS | Mn2+, DVDMS | 3D | 197 | DVDMS | - | - | - | pH/GSH | 630 | MCF-7 | PTT-PDT | 114 | |
| Cu-TCPP | Cu2+, TCPP | 3D | 200 | Cu2+/TCPP | - | - | - | - | 808/660 | Saos-2 | PTT-PDT | 85 | |
| Cu10MOF | Zr4+, TCPP | 3D | 100 | Cu2+/TCPP | 10% | - | - | - | 660 | NIH 3T3 | PTT-PDT | 86 | |
| Zn-TCPP | Zn2+, TCPP | 3D | - | Zn2+/TCPP | - | - | - | - | 650 | Hela | PTT-PDT | 87 | |
| AuNR@MOFs | Zr4+, TCPP | 3D | 103×187 | AuNR/TCPP | - | - | - | - | 640/808 | 4T1 | PTT-PDT | 88 | |
| Fe-DSCP-PEG-cRGD | Fe3+, DSCP | 3D | 51 | Fe2+/cisplatin | - | PEG | cRGD | GSH | - | C6 | CT-CDT | 25 | |
| DSF/DOX@ZIF-8@Cu-TA | Zn2+, 2-MIm | 3D | 99 | DOX/CuL2/Cu+ | 10.56% | - | - | pH | - | MDA-MB-231/L02/L929 | CT-CDT | 115 | |
| PCN-224(Cu)-GOD@MnO2 | Zr4+, TCPP | 3D | 178 | GOx/TCPP | 42.5% | MnO2 | - | - | - | HeLa | CT-CDT | 116 | |
| MGDFT | Fe3+, H2BDC | 3D | 200 | DOX/Fe2+ | - | - | NH2-PEG-FA/TPP | Phosphate | - | 4T1/A549/293T | CT-CDT | 89 | |
| hMIL-88B(Fe)@ZIF-8-MnOx-DOX-FA | Fe3+, NH2-BDC | 3D | 102× 196 | DOX/Fe2+ | 43.2% | - | FA | pH | - | MCF-7/HepG-2/hCMEC-D3 | CT-CDT | 90 | |
| O2-Cu/ZIF-8@Ce6/ZIF-8@F127 | Zn2+, 2-mim | 3D | 95 | Ce6/Cu+ | 3.3% | F127 | - | pH | 650 | 4T1 | PDT-CDT | 91 | |
| Zr-Fc | Zr4+, Fc(COOH)2 | 3D | - | Fc(COOH)2 | - | - | - | - | 808 | 7702/4T1/Huh7 | PTT-CDT | 92 | |
| FeS2-PEG | Fe3+ | 3D | 180 | Fe2+/Fe2O3 | - | PEG | - | H2O2 | 808 | 4T1 | PTT-CDT | 117 | |
| HG-MIL@PDA | Fe3+, NH2-H2BDC | 3D | 233 | PDA/Fe2+ | - | - | HA | pH/NIR | 808 | MDA-MB-231/L02 | PTT-CDT | 118 | |
| AuNR@MOFs@CPT | Zr4+, TCPP | 3D | 25×53 | AuNR/CPT/TCPP | 25% | - | - | NIR | 808/660 | 4T1 | CT-PTT-PDT | 93 | |
| MIL-88-ICG@ZIF-8-DOX | Zr4+, TCPPFe3+, NH2-BDC | 3D | 450 | DOX/ICG | 25.3% | - | - | pH | 808 | 4T1 | CT-PTT-PDT | 119 | |
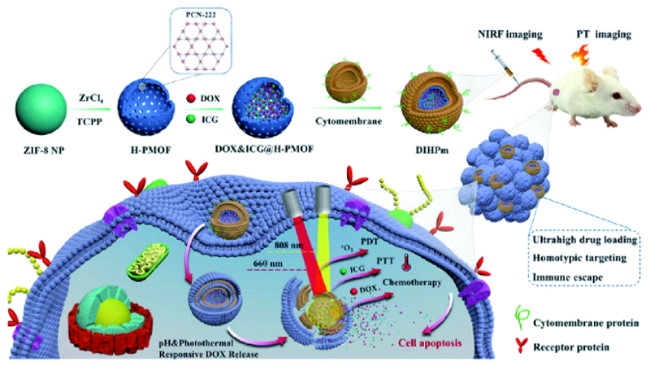
| DOX&ICG@H-PMOFs@mem | Zr4+, TCPP | 3D | 235 | DOX/ICG/TCPP | 635% | mem | mem | pH/NIR | 808/660 | 4T1/U87MG/A549 | CT-PTT-PDT | 94 | |
| DOX-Pt-tip-ped Au@ZIF-8 | Zn2+, 2-mim | 3D | 150 | DOX | 24.9% | - | PEG-FA | pH | 1064 | 4T1/HeLa | CT-PTT-PDT | 120 | |
| ICG@Mn/Cu/Zn-MOF@MnO2 | Cu+, Zn2+, 2-ICA | 3D | 220 | ICG/Cu+/Mn2+ | 56.8% | MnO2 | - | GSH | 808 | U87/NIH-3T3 | CDT-PTT-PDT | 95 | |