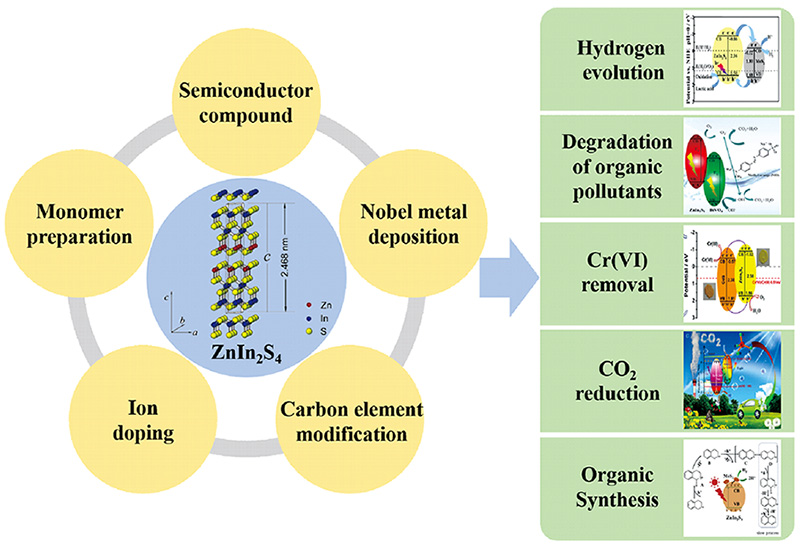
1 引言
1.1 光催化技术简介
图1 (a)半导体光催化原理的示意图[7], (b)六方相[8]和(c)立方相ZnIn2S4[9]的晶体结构, (d)六方相和(e)立方相ZnIn2S4的能带结构[10]Fig.1 (a) Schematic illustration of the principle of semi-conductor photocatalysis[7], crystal structures of (b) hexagonal[8] and (c) cubic ZnIn2S4[9], energy band structure of (d) hexagonal and (e) cubic ZnIn2S4[10] |
1.2 硫化铟锌的性质
2 硫化铟锌的合成及改性
2.1 硫化铟锌单体的合成
图2 (a)ZnIn2S4纳米管和纳米带的生长机理示意图[8], (b)在CTAB和PEG-6000存在下获得的ZnIn2S4微球和纳米线的生长机理示意图[8]Fig.2 (a) Schematic illustration of the growth mechanism for ZnIn2S4 nanotubes and nanoribbons[8], (b) Schematic illustration of the growth mechanism of ZnIn2S4 microsphere and nanowires obtained in the presence of CTAB and PEG-6000[8] |
2.2 构建复合半导体
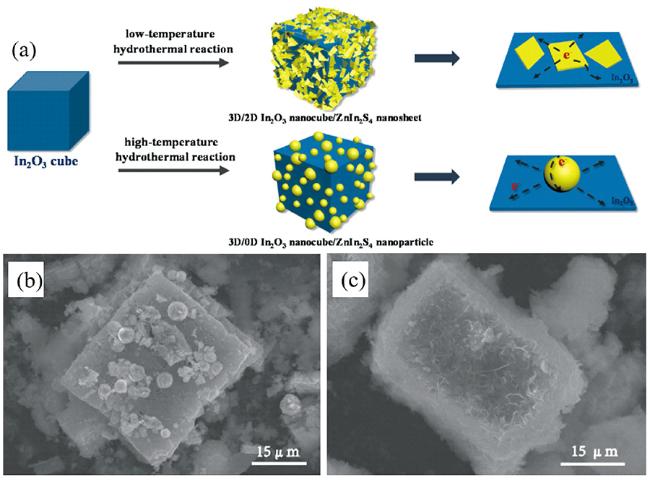
图3 (a)In2O3纳米立方体/ZnIn2S4纳米片和In2O3纳米立方体/ZnIn2S4纳米颗粒的制备示意图, (b)In2O3纳米立方体/ZnIn2S4纳米片和(c)In2O3纳米立方体/ZnIn2S4纳米颗粒的SEM图[37]Fig.3 (a) Schematic of the preparation for In2O3 nanocube/ZnIn2S4 nanosheet and In2O3 nanocube/ZnIn2S4 nanoparticle, SEM images of (b) In2O3 nanocube/ZnIn2S4 nanosheet and (c) In2O3 nanocube/ZnIn2S4 nanoparticle[37] |
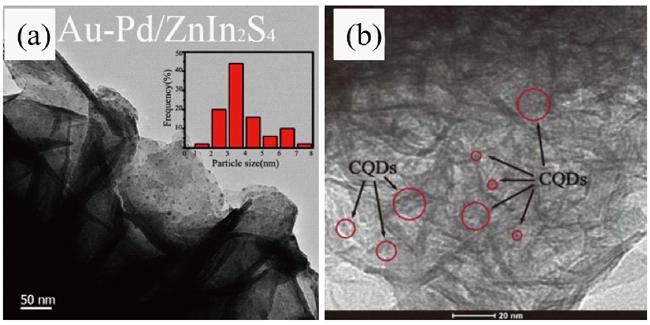
2.3 贵金属沉积
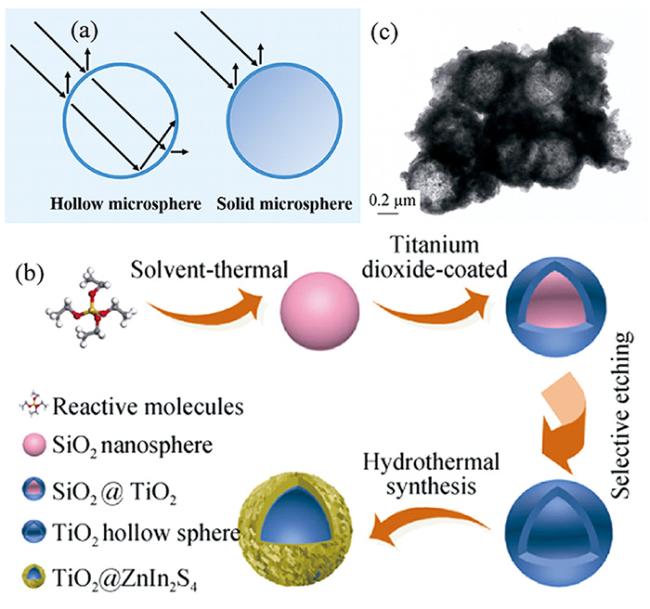
2.4 碳单质修饰
2.5 离子掺杂
表1 不同ZnIn2S4基光催化剂的产氢性能的研究Table 1 Examples of hydrogen production performance by different ZnIn2S4-based photocatalysts |
| Photocatalyst | Hydrogen production rate | Lighting conditions | Sacrificial reagents | ref |
|---|---|---|---|---|
| ZnIn2S4 ultra-thin nanosheet | 1.94 mmol/g/h | 300 W Xenon lamp, λ≥420 nm | TEOA | 16 |
| ZnIn2S4 ultra-thin nanosheet with sulfur vacancies | 13.478 mmol/g/h | 500 W Xenon lamp, λ≥400 nm | TEOA | 15 |
| MoS2/ZnIn2S4 | 4287.5 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | Lactic acid | 54 |
| MoS2/ZnIn2S4 | 2512.5 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | Lactic acid | 53 |
| MoS2/ZnIn2S4 | 8898 μmol/g/h | 300 W Xenon lamp, λ≥400 nm | TEOA | 86 |
| WS2/ZnIn2S4 | 293.3 μmol/g/h | 150 W Xenon lamp, λ≥400 nm | NaS2/Na2SO3 | 34 |
| WS2/ZnIn2S4 | 2.55 mmol/g/h | 300 W Xenon lamp, λ≥420 nm | Lactic acid | 62 |
| WS2/ZnIn2S4 | 199.1 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 61 |
| NiS/ZnIn2S4 | 3.3 mmol/g/h | 320 W Xenon lamp, λ≥420 nm | Lactic acid | 57 |
| NiS/ZnIn2S4 | 2094 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 56 |
| AgIn5S8/ZnIn2S4 | 949 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 65 |
| g-C3N4/ZnIn2S4 | 7740 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | TEOA | 69 |
| Au/thiol-UiO66/ZnIn2S4 | 3916 μmol/g/h | 300 W Xenon lamp, λ: 420~780 nm | NaS2/Na2SO3 | 73 |
| ZnIn2S4/NH2-MIL-125(Ti) | 2204.2 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 76 |
| TiO2/ZnIn2S4 hollow structure | 1129.5 μmol/g/h | 300 W Xenon lamp, visible light | Lactic acid | 84 |
| Co9S8/ZnIn2S4 hollow structure | 6250 μmol/g/h | 300 W Xenon lamp, λ≥400 nm | TEOA | 64 |
| 2D/2D MoS2/ZnIn2S4 | 4.974 mmol/g/h | 300 W Xenon lamp, visible light | Lactic acid | 55 |
| 2D/2D CuInS2/ZnIn2S4 | 3430.2 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 66 |
| MoS2/CQDs/ZnIn2S4 | 3 mmol/g/h | 300 W Xenon lamp, λ≥420 nm | TEOA | 113 |
| NiS/CQDs/ZnIn2S4 | 568 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | TEOA | 114 |
| WO3/ZnIn2S4 | 2202.9 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 42 |
| Cu3P/ZnIn2S4 | 2561.1 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 80 |
| RGO/ZnIn2S4 | 1210 μmol/g/h | 350 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 103 |
| RGO/ZnIn2S4 | 817 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | Lactic acid | 104 |
| Ca-Doped ZnIn2S4 | 692 μmol/g/h | 300 W Xenon lamp, λ≥430 nm | NaS2/Na2SO3 | 107 |
| Cu-Doped ZnIn2S4 | 757.5 μmol/g/h | 300 W Xenon lamp, λ≥430 nm | NaS2/Na2SO3 | 110 |
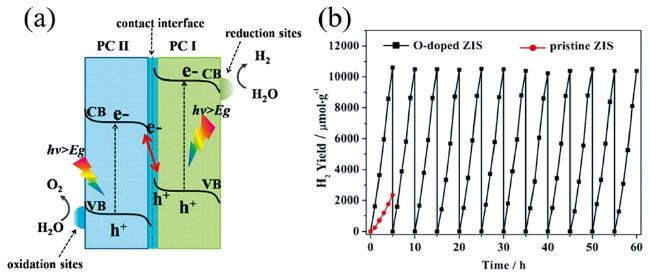
| Oxygen-Doped ZnIn2S4 | 2120 μmol/g/h | 300 W Xenon lamp, λ≥420 nm | NaS2/Na2SO3 | 112 |
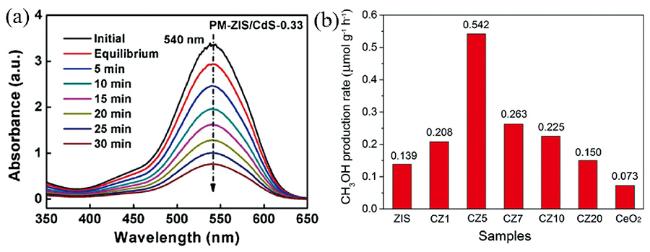
3 硫化铟锌在光催化领域的应用
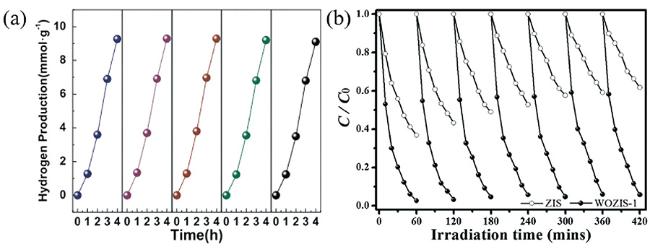
3.1 光催化产氢
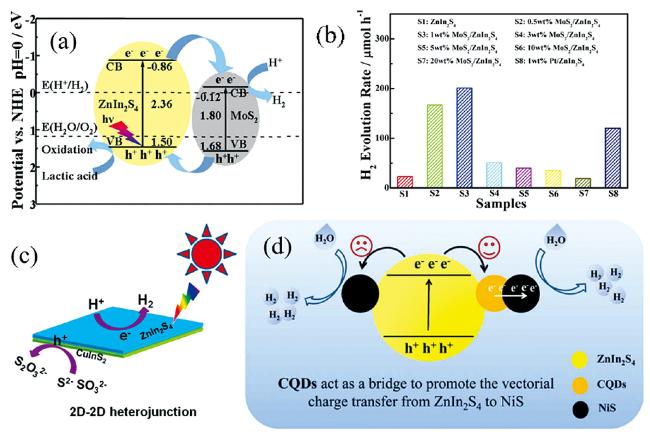
图6 (a) MoS2/ZnIn2S4的光催化产氢机理[54], (b) MoS2/ZnIn2S4光催化产氢性能[53], (c) 2D/2D CuInS2/ZnIn2S4的光催化产氢机理[66], (d) NiS/CQDs/ZnIn2S4的光催化产氢机理[114]Fig.6 (a) Photocatalytic H2 evolution mechanism of the MoS2/ZnIn2S4[54], (b) Photocatalytic H2 evolution performance of the MoS2/ZnIn2S4[53], (c) Photocatalytic H2 evolution mechanism of 2D/2D CuInS2/ZnIn2S4[66], (d) Photocatalytic H2 evolution mechanism of the NiS/CQDs/ZnIn2S4[114] |
表2 不同ZnIn2S4基光催化剂的降解性能的研究Table 2 Examples of degradation performance by different ZnIn2S4-based photocatalysts |
| Photocatalyst | Organic Pollutants | Degradation efficiency | Lighting conditions | ref |
|---|---|---|---|---|
| g-C3N4/ZnIn2S4 (20 mg) | MO (50 mL, 10 mg/L) | 95.3% (120 min) | 500 W Xenon lamp, λ≥420 nm | 70 |
| g-C3N4/ZnIn2S4 (20 mg) | Phenol (50 mL, 10 mg/L) | 72.3% (240 min) | 500 W Xenon lamp, λ≥420 nm | 70 |
| g-C3N4/ZnIn2S4 (50 mg) | TC (100 mL, 20 mg/L) | 100% (120 min) | 300 W Xenon lamp, λ≥400 nm | 71 |
| BiPO4/ZnIn2S4 (15 mg) | TC (50 mL, 40 mg/L) | 84% (90 min) | 300 W Xenon lamp, visible light | 119 |
| MoS2/ZnIn2S4 (10 mg) | MO (10 mL, 20 mg/L) | 90% (60 min) | 300 W Xenon lamp, λ≥400 nm | 51 |
| TiO2/ZnIn2S4 film (2*2 cm2) | MB (5 mL, 3 mg/L) | 91% (4 h) | 100 W Incandescent lamp | 120 |
| CdIn2S4/ZnIn2S4 (40 mg) | MO (80 mL, 10 mg/L) | 99.7% (90 min) | 500 W Halogen lamp, λ≥420 nm | 89 |
| CdIn2S4/ZnIn2S4 (40 mg) | RhB (80 mL, 10 mg/L) | 100% (70 min) | 500 W Halogen lamp, λ≥420 nm | 89 |
| TiO2/ZnIn2S4 (30 mg) | Carbamazepine (400 mL, 100 mg/L) | 100% (4 h) | Sunlight, λ≥400 nm | 38 |
| In2O3/ZnIn2S4 (25 mg) | 2,4-dichlorophenol (50 mL, 20 mg/L) | 95.8% (120 min) | 300 W Xenon lamp, λ≥420 nm | 37 |
| MIL-88A(Fe)@ZnIn2S4 (mg) | SMZ (40 mL, 20 mg/L) | 99.6% (60 min) | 500 W Xenon lamp | 74 |
| TiO2/ZnIn2S4 hollow structure (20 mg) | LEV (80 mL, 10 mg/L) | 81.07% (4 h) | 250 W Xenon lamp, λ≥400 nm | 85 |
| TiO2/ZnIn2S4 hollow structure (20 mg) | TC (80 mL, 10 mg/L) | 82.74% (90 min) | 250 W Xenon lamp, λ≥400 nm | 85 |
| TiO2/ZnIn2S4 hollow structure (20 mg) | RhB (80 mL, 20 mg/L) | 98.41% (60 min) | 250 W Xenon lamp, λ≥400 nm | 85 |
| 2D/2D BiOCl/ZnIn2S4 (200 mg) | Phenol (200 mL, 20 mg/L) | 77.4% (6 h) | 300 W Xenon lamp, λ≥400 nm | 81 |
| 2D/2D g-C3N4/ZnIn2S4 (20 mg) | TC (50 mL, 50 mg/L) | 85% (120 min) | 500 W Xenon lamp, λ≥420 nm | 82 |
| CQDs/BiOCl/ZnIn2S4 (50 mg) | TC (100 mL, 10 mg/L) | 83.7% (2 h) | 300 W Xenon lamp, λ≥400 nm | 121 |
| AgPO4/g-C3N4/ZnIn2S4 (50 mg) | TC (100 mL, 20 mg/L) | 83% (60 min) | 300 W Xenon lamp, λ≥400 nm | 122 |
| WO2.72/ZnIn2S4 (30 mg) | TC (30 mL, 50 mg/L) | 97.3% (60 min) | 300 W Xenon lamp, λ≥400 nm | 123 |
| Bi2S3/ZnIn2S4 (50 mg) | MB (100 mL, 40 mg/L) | 95.4% (150 min) | 30 W Xenon lamp, visible light | 63 |
| Au-MoS2/ZnIn2S4 (10 mg) | Phenol (10 mL, 20 mg/L) | 84% (60 min) | Sunlight | 87 |
| Bi2WO6/ZnIn2S4 (100 mg) | MTZ (500 mL, 10 mg/L) | 56% (250 min) | 500 W Halogen lamp | 45 |
| MO3/ZnIn2S4 (200 mg) | MO (200 mL, 30 mg/L) | 98% (100 min) | 500 W Halogen lamp, λ≥420 nm | 48 |
| MO3/ZnIn2S4 (200 mg) | RhB (200 mL, 10 mg/L) | 99% (80 min) | 500 W Halogen lamp, λ≥420 nm | 48 |
| BiVO4/ZnIn2S4 (20 mg) | MO (100 mL, 15 mg/L) | 86% (240 min) | 300 W LED lamp, visible light | 44 |
| CQDs/ZnIn2S4 (50 mg) | MO (100 mL, 10 mg/L) | 100% (40 min) | 300 W Xenon lamp, λ≥420 nm | 101 |
| CQDs/ZnIn2S4 (20 mg) | TC (80 mL, 10 mg/L) | 85.07% (90 min) | 250 W Xenon lamp, λ≥420 nm | 100 |
| Sm-Doped ZnIn2S4 (50 mg) | RhB (50 mL, 20 mg/L) | 100% (90 min) | 400 W Xenon lamp, λ≥420 nm | 106 |
3.2 光催化降解有机污染物
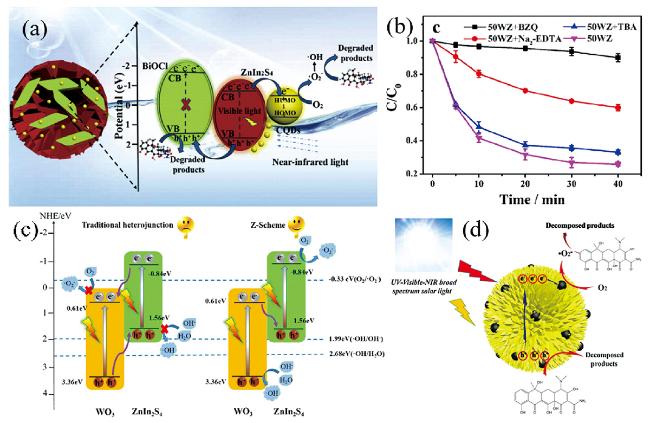
图8 (a) CQDs/BiOCl/ZnIn2S4的光催化降解机理[121], (b) 乙炔吡喃在WO3/ZnIn2S4上光催化降解活性自由基的捕获实验[127], (c) WO3/ZnIn2S4的光催化活性增强的机理[127], (d) CQDs/ZnIn2S4的光催化活性增强的机理[100]Fig.8 (a) Photocatalytic degradation mechanism of the CQDs/BiOCl/ZnIn2S4[121], (b) Trapping experiment of active species during the photocatalytic degradation of nitenpyram over WO3/ZnIn2S4[127], (c) Mechanism of the enhanced photocatalytic activity of WO3/ZnIn2S4[127], (d) Mechanism of the enhanced photocatalytic activity of CQDs/ZnIn2S4[100] |