1 引言
2 金属基催化剂催化的苯羟基化反应
表1 不同反应机理的苯羟基化反应金属基催化剂的活性对比Table 1 Comparison of activities of metal-based catalysts for hydroxylation of benzene via different reaction mechanism |
| Mechanism | Catalyst | Reaction condition | Activitya | ref |
|---|---|---|---|---|
| H2O2-mediated radical | NaVO3 | C6H6 11.3 mmol, H2O2 19.4 mmol, catalyst 0.2 mmol, 25 ℃, 13 h | Y 13.5%, S 94.0% | 38 |
| [Cu2(μ-OH)(6-hpa)](ClO4)3 | C6H6 60 mmol, H2O2 120 mmol, catalyst 1 μmol,50 ℃, 40 h | X 22.0%, S 95.2% | 12 | |
| Fe(DS)3 | C6H6 11.3 mmol, H2O2 11.3 mmol, catalyst 0.05 mmol, 50 ℃, 6 h | Y 54.0%, S 100% | 40 | |
| [Dmim]2.5PMoV | C6H6 10 mmol, H2O2 30 mmol, catalyst 100 mg, 70 ℃, 4 h | Y 26.5%, S 100% | 25 | |
| [C3CNpy]4HPMoV2 | C6H6 10 mmol, H2O2 30 mmol, catalyst 100 mg, 60 ℃, 2 h | Y 31.4%, S 95.8% | 27 | |
| P-[DVB-VBIM]5PMoV2 | C6H6 10 mmol, H2O2 30 mmol, catalyst 100 mg, 55 ℃, 6 h | Y 23.7%, S 100% | 41 | |
| [VO(acac)2]- grafted PMO | C6H6 4 mmol, H2O2 12 mmol, catalyst 300 mg, 50 ℃, 8 h | X 27.4%, S 100% | 19 | |
| POM@OMP | C6H6 6 mmol, H2O2 18 mmol, catalyst 50 mg, 80 ℃, 10 h | Y 33.0%, S 100% | 42 | |
| Cu2O-rGO | C6H6 1 mmol, H2O2 2 mmol, catalyst 1 mg, 40 ℃, 12 h | Y 21.2%, S 85.5% | 22 | |
| FeOCl | C6H6 10 mmol, H2O2 10 mmol, catalyst 100 mg, 60 ℃, 4 h | Y 43.5%, S 100% | 23 | |
| H2O2-mediated non-radical | [NiⅡ(tepa)]2+ | C6H6 5 μmol, H2O2 2.5 mmol, catalyst 0.5 μmol, 60 ℃, 5 h | Y 21.0%, S 91.3% | 43 |
| [CoⅡ(L3)Cl]Ph4B | C6H6 5 mmol, H2O2 25 mmol, catalyst 5 μmol, 60 ℃, 5 h | Y 29.0%, S 97.0% | 44 | |
| Fe-N4 | C6H6 4.5 mmol, H2O2 55 mmol, catalyst 50 mg, 30 ℃, 24 h | Y 78.4%, S 100% | 45 | |
| V-Si-ZSM-22 | C6H6 5 mmol, H2O2 5 mmol, catalyst 100 mg, 80 ℃, 30 s | Y 30.8%, S> 99% | 13 | |
| O2-mediated radical | VxOy@C-S | C6H6 11.3 mmol, O2 3.0 MPa, catalyst 50 mg, ascorbic acid 0.8 g, 80 ℃, 4 h | Y 9.3%, S 96.0% | 9 |
| V/UiO-66-NH2 | C6H6 11.3 mmol, O2 3.0 MPa, catalyst 50 mg, ascorbic acid 0.7 g, 60 ℃, 21 h | Y 22.0%, S 98.1% | 46 | |
| VOC2O4-N-5 | C6H6 11.3 mmol, O2 1.0 MPa, catalyst 100 mg, 150 ℃, 10 h | X 4.2%, S 96.3% | 47 | |
| O2-mediated non-radical | H7PMo8V4O40 | C6H6 2 mmol, Air 1.5 MPa, catalyst 55 mg, CO 0.5 MPa, 90 ℃, 15 h | Y 28.1%, S 59.3% | 28 |
| POM@MOF@SBA-15 | C6H6 10 mmol, O2 2.0 MPa, catalyst 200 mg,ascorbic acid 0.9 g, 80 ℃, 20 min | Y 6.0%, S> 99% | 48 | |
| PMoV@PCIF-1 | C6H6 22.5 mmol, O2 2.0 MPa, catalyst 300 mg, ascorbic acid 0.8 g, 100 ℃, 10 h | Y 12.0%, S 100% | 49 | |
| PdII(bpym) | C6H6 5.6 mmol, O2 2.0 MPa, catalyst 0.02 mmol,Al(OTf)3 0.04 mmol, 100 ℃, 16 h | Y 3.7%, S 79.7% | 31 | |
| H5PV2Mo10O40 | C6H6 0.5 mmol, O2 1.0 MPa, catalyst 800 mg, 50% H2SO4 5 mL, 170 ℃, 6 h | X 65.0%, S 95.0% | 50 | |
| O2-mediated synergistic catalysis | HMS-HPA(V2)+ Pd(OAc)2 | C6H6 22.5 mmol, O2 2.0 MPa, catalyst 500 mg+10 mg, LiOAc 0.2 g, 120 ℃, 10 h | X 12.2%, S 75.6% | 29 |
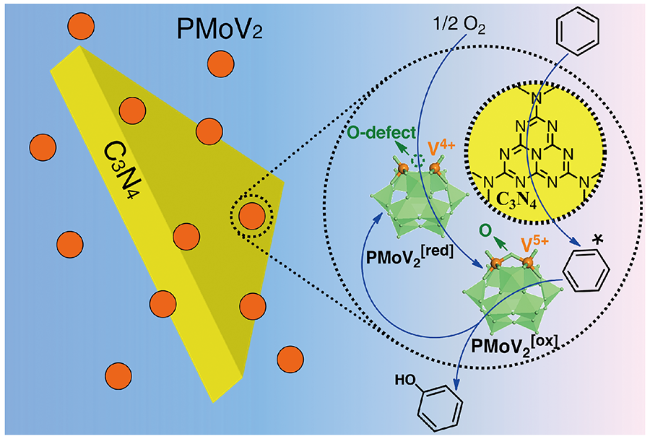
| C3N4(580)+PMoV2 | C6H6 45 mmol, O2 2.0 MPa, catalyst 100 mg+400 mg, LiOAc 0.6 g, 130 ℃, 4.5 h | Y 13.6%, S 100% | 26 | |
| g-C3N4+Ch5PMoV2 | C6H6 5 mmol, O2 2.0 MPa, catalyst 12 mg+30 mg,LiOAc 0.06 g, 120 ℃, 4.5 h | Y 10.7%, S> 99% | 51 | |
| SFNC(800)+Ch5PMoV2 | C6H6 5 mmol, O2 2.0 MPa, catalyst 10 mg+30 mg,LiOAc 0.06 g, 120 ℃, 3 h | Y 11.2%, S> 99% | 52 | |
| [DiBimCN]2HPMoV2 @NC580 | C6H6 45 mmol, O2 2.2 MPa, catalyst 550 mg, LiOAc 0.6 g,140 ℃, 17 h | Y 10.5%, S 100% | 53 | |
| FeC(5) | C6H6 45 mmol, O2 2.2 MPa, catalyst 200 mg,LiOAc 0.6 g, 150 ℃, 30 h | Y 14.2%, S 100% | 54 |
a Activity includes Y (or X) and S. Y is yield for phenol, X is conversion of benzene, and S is selectivity for phenol based on the formed phenol plus byproducts. |
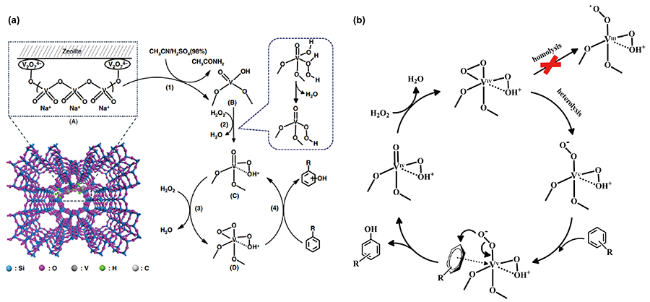
2.1 H2O2为氧化剂的自由基反应机理
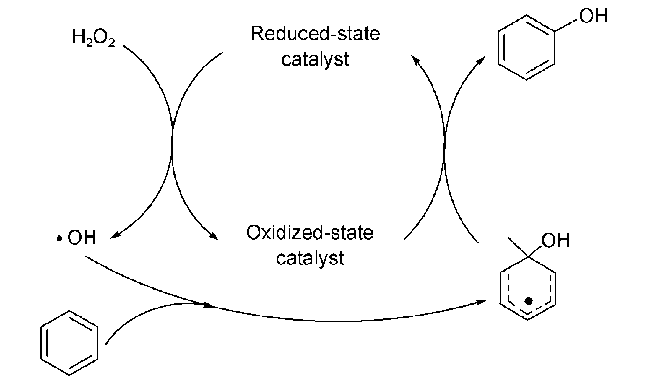
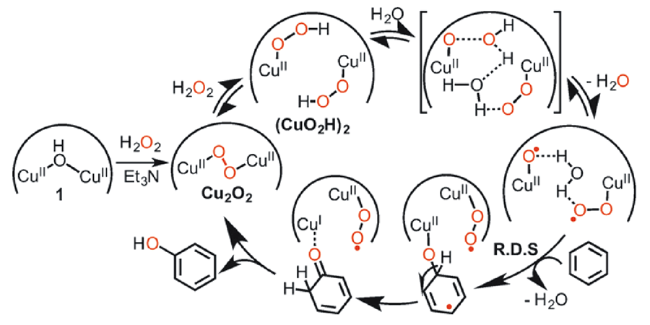
2.2 H2O2为氧化剂的非自由基反应机理
 进攻苯环,随后中间体发生氧转移与苯环生成C—O键,最后通过氢转移得到苯酚。一般情况下,氧转移步骤(即C—O键的生成)为非自由基机理的速率决定步骤[45,61]。非自由基机理能促使芳香 —H键的快速活化,并抑制酚类产物的过度氧化,是提高苯羟基化反应活性与选择性的有效方法。
进攻苯环,随后中间体发生氧转移与苯环生成C—O键,最后通过氢转移得到苯酚。一般情况下,氧转移步骤(即C—O键的生成)为非自由基机理的速率决定步骤[45,61]。非自由基机理能促使芳香 —H键的快速活化,并抑制酚类产物的过度氧化,是提高苯羟基化反应活性与选择性的有效方法。2.3 O2为氧化剂的自由基反应机理
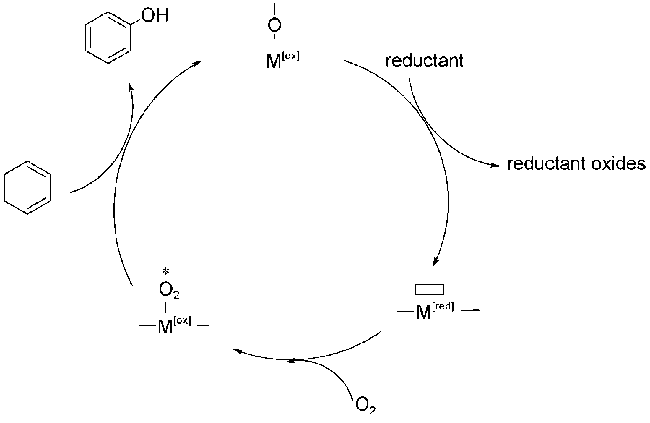
2.4 O2为氧化剂的非自由基反应机理
2.5 O2为氧化剂的双活性中心协同催化机理
 -Fe2+,最终氧气填补到Fe2+-
-Fe2+,最终氧气填补到Fe2+-  -Fe2+中再生氧缺位。在无还原剂条件下,FeC(5)催化苯环羟基化得到14.2%的苯酚收率,催化剂在反应结束后经过滤即可回收,且复用性能较好。
-Fe2+中再生氧缺位。在无还原剂条件下,FeC(5)催化苯环羟基化得到14.2%的苯酚收率,催化剂在反应结束后经过滤即可回收,且复用性能较好。3 非金属材料催化的苯羟基化反应
表2 非金属材料催化的苯羟基化反应活性对比Table 2 Comparison of activities of non-metal catalysts for hydroxylation of benzene |
| Catalyst | Reaction condition | Activitya | ref |
|---|---|---|---|
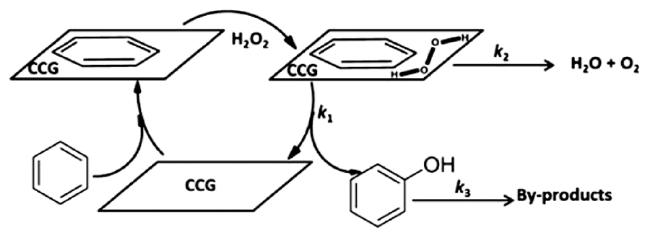
| CCG | C6H6 1.67 mmol, H2O2 22.5 mmol, catalyst 20 mg,60 ℃, 16 h | X 18.5%, S 99% | 11 |
| MWCNTs | C6H6 11.3 mmol, H2O2 13.5 mmol, catalyst 50 mg,60 ℃, 2.5 h | X 7.0%, S 97% | 83 |
| CNT7000 | C6H6 11.3 mmol, H2O2 22.5 mmol, catalyst 100 mg, 60 ℃, 36 h | Y 13.7% | 84 |
| HPC-400 | C6H6 130 g, H2O2 22.5 mmol, catalyst 20 mg, 60 ℃, 16 h | X 4.1%, S 99% | 85 |
| WAC-0.5N-8H | C6H6 22.5 mmol, H2O2 58.8 mmol, catalyst 600 mg, 70 ℃, 6 h | Y 13.5%, S 86.3% | 86 |
| NOC-0.15 | C6H6 45 mmol, O2 2.2 MPa, catalyst 200 mg,LiOAc 0.6 g, 150 ℃, 48 h | Y 12.5%, S>99% | 72 |
| AC-70 | C6H6 45 mmol, O2 2.2 MPa, catalyst 200 mg, LiOAc 0.6 g, 155 ℃, 36 h | Y 10.1%, S 100% | 87 |
| QAP | C6H6 5.7 mmol, O2 2.0 MPa, catalyst 100 mg, LiOAc 0.4 g, 120 ℃, 12 h | Y 15.9%, S>99% | 88 |
a Activity includes Y (or X) and S. Y is yield for phenol, X is conversion of benzene, and S is selectivity for phenol based on the formed phenol plus byproducts. |
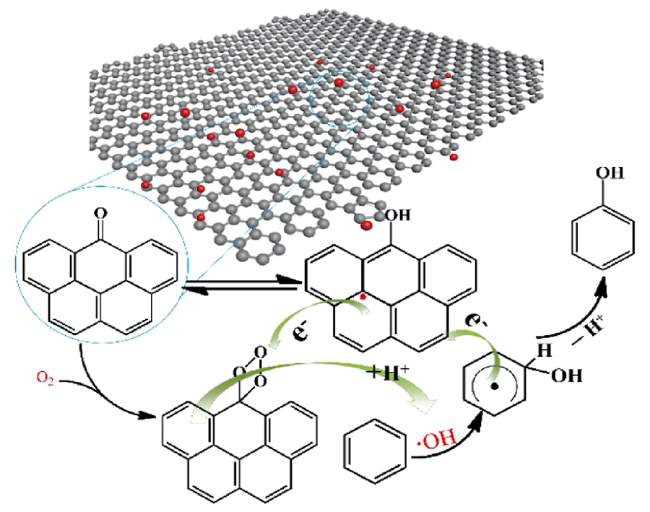
3.1 碳材料催化苯羟基化反应的自由基机理
3.2 高聚物催化苯羟基化反应的自由基机理
4 结论与展望
附表 催化剂缩写的英文全称说明Supplementary Table Abbreviation for catalyst and corresponding English full name |
| Abbreviation for catalysta | English full name |
|---|---|
| AC | activated carbon |
| bpym | 2,2'-bipyrimidine |
| C3CNpy | N-butyronitrile pyridine |
| CCG | chemically converted graphene |
| CNT | carbon nanotube |
| Dmim | 1,1-(butane-1,4-diyl)-bis(3- methylimidazolium) |
| DiBimCN | 4,4'-butyl-bis(3-cyanopropyl-imidazole) |
| Fe(DS)3 | Ferric tri(dodecane sulfonate) |
| 6-hpa | 1,2-bis[2-[bis(2-pyridylmethyl)aminomethyl] -6-pyridyl]ethane |
| HMS | hexagaonal mesoporous silica |
| HPA | heteropolyacid |
| HPC | honeycomb-like porous carbon |
| L3 | N1,N1-bis((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)-N3,N3-dimethylpropane-1, 3-diamine |
| MOF | metal organic framework |
| MWCNTs | multi-walled carbon nanotubes |
| NOC | N-doped and surface O-enriched mesoporous carbons |
| OMP | ordered mesoporous polymer |
| P-DVB-VBIM | poly divinylbenzene- 3-n-butyl- 1-vinylimidazolium |
| PMoV | H3+xPMo12-xVxO40 |
| PMO | periodic mesoporous organosilica |
| POM | polyoxometalate |
| PCIF | porous cationic framework |
| QAP | quinone-amine polymer |
| rGO | reduced graphene oxide |
| SNFC | soybean flour prepared nitrogen-doped carbon |
| tepa | tris[2-(pyridin-2-yl)ethyl]amine |
| V-Si-ZSM | V-containing all-silica zeolite |
| WAC | wood-based activated carbon |
a With an alphabetical order of the first letter in abbreviations. |





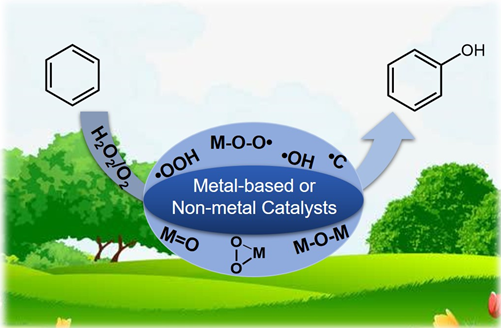
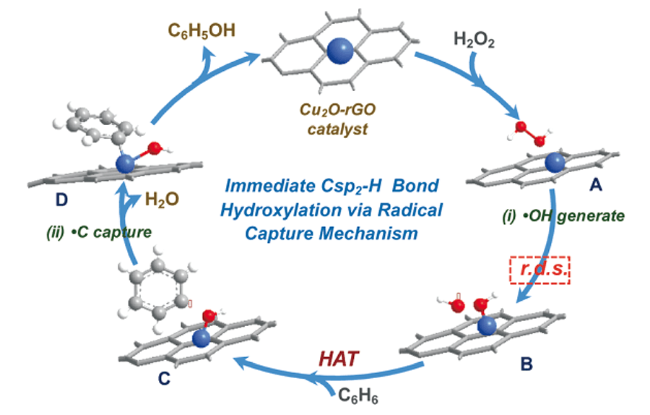
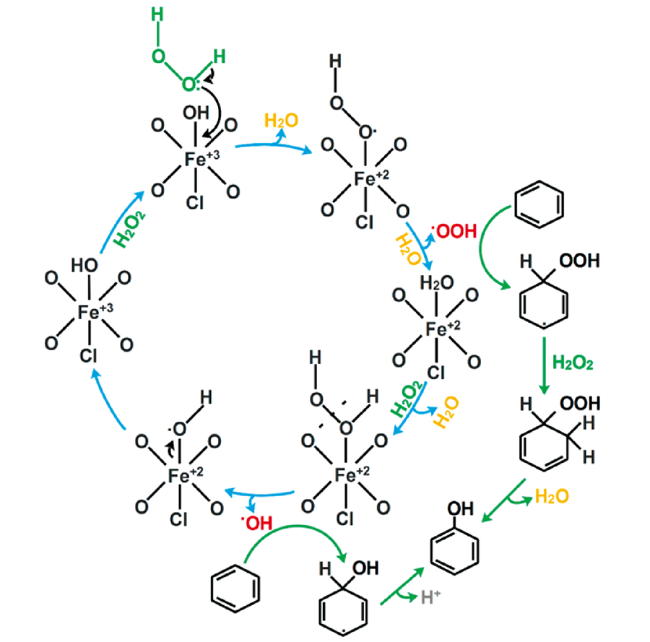
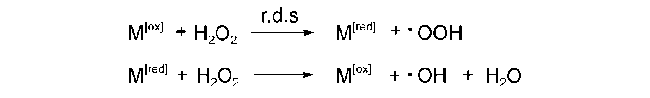

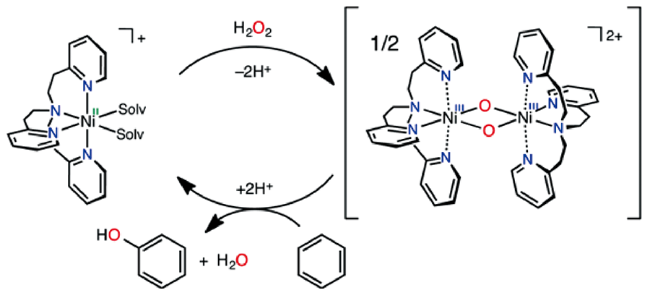
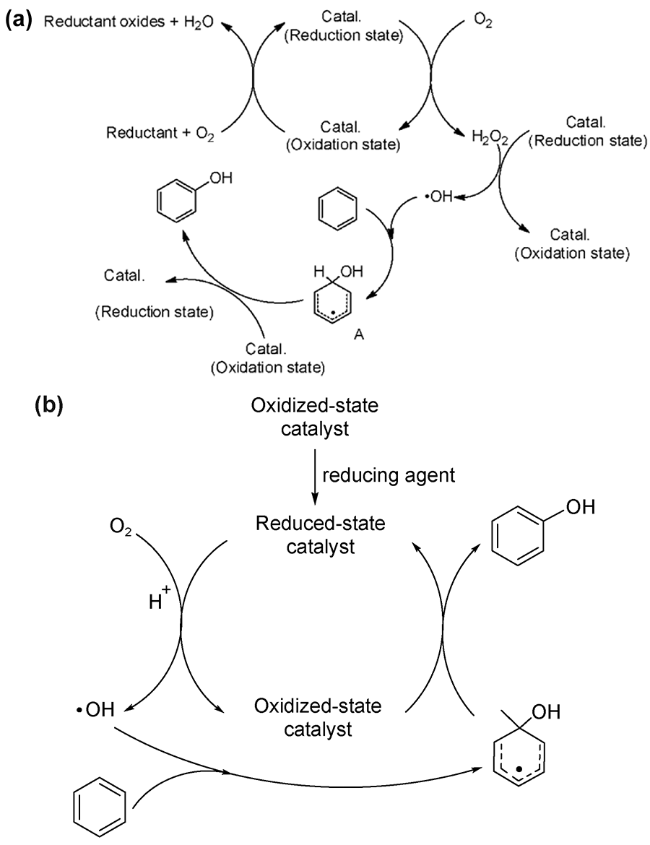

 ;O2被活化填充到表面氧缺位生成金属-氧活性中间体
;O2被活化填充到表面氧缺位生成金属-氧活性中间体