1 引言
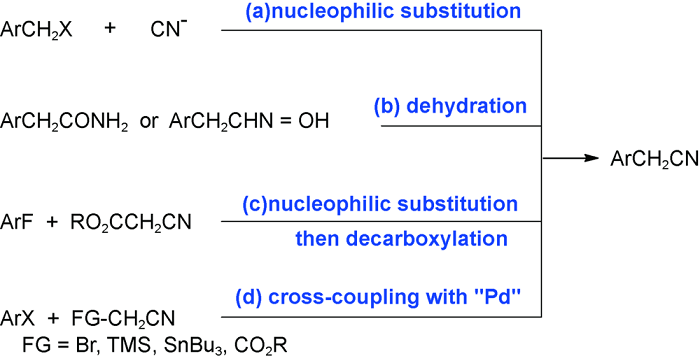
2 乙腈对活化烯烃的自由基氰甲基化
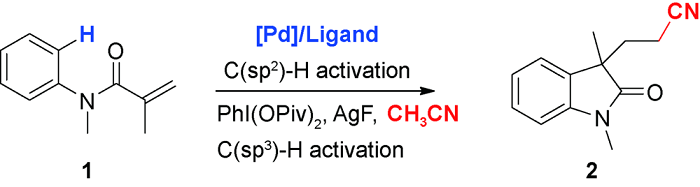
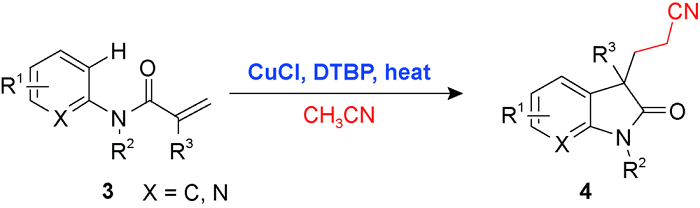
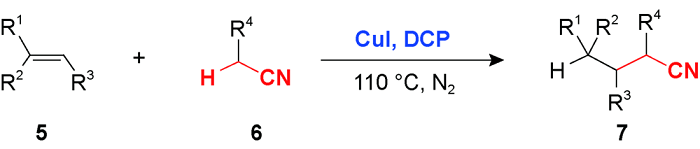
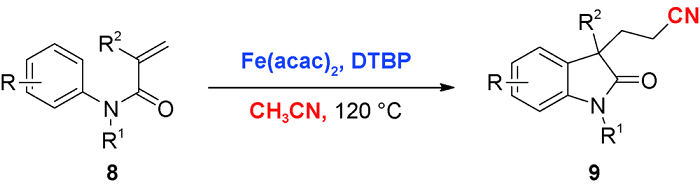
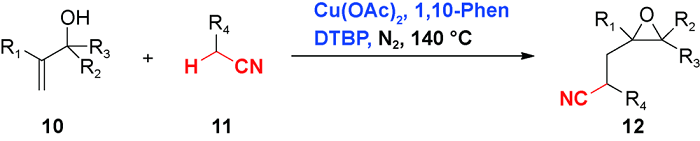
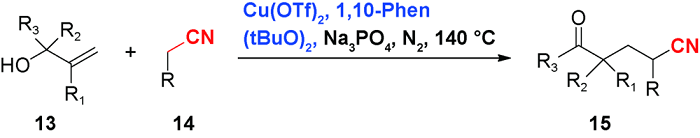
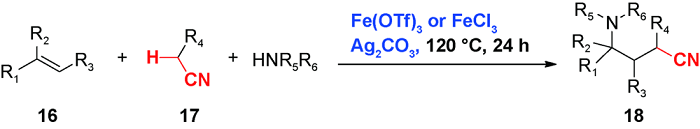
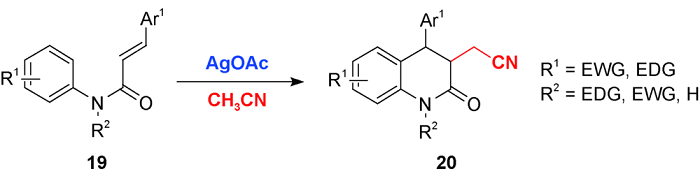
3 乙腈对烯烃或芳香C(sp2)—H官能化的光化学偶联反应
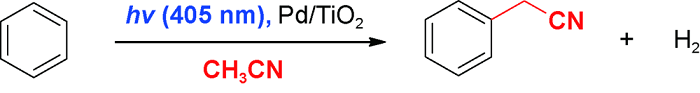
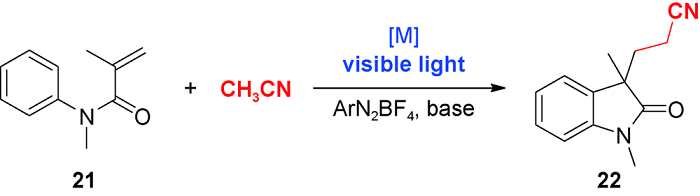
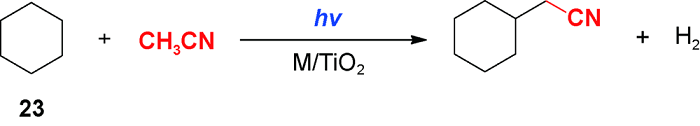
4 乙腈对芳环C(sp2)—H官能化的脱氢偶联反应
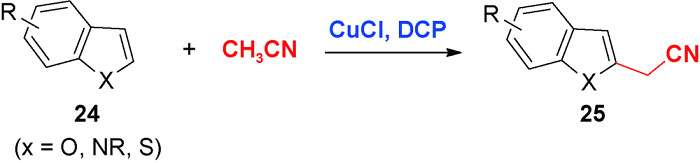
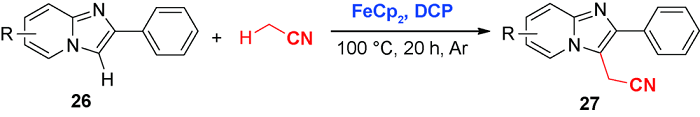
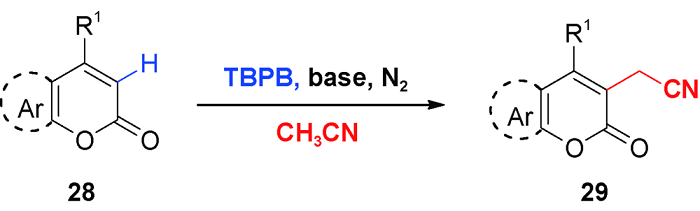
5 导向基团促进的C—H氰甲基化
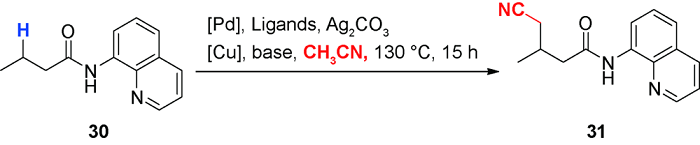
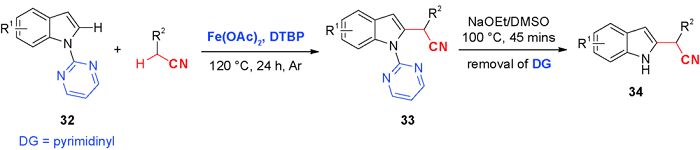
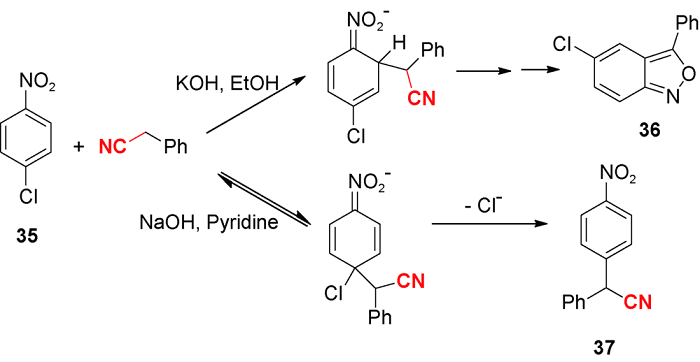
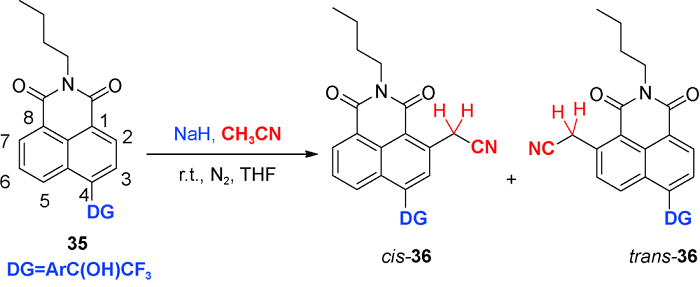
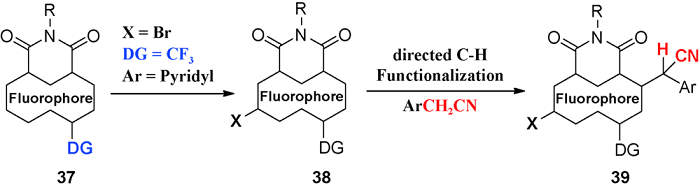
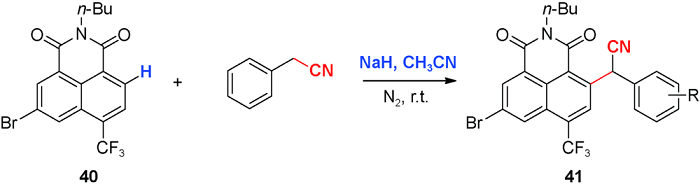

C—H氰烷基化:导向基控制的萘酰亚胺C—H氰烷基化
收稿日期: 2020-10-09
修回日期: 2021-01-14
网络出版日期: 2021-03-04
基金资助
上海市科学技术委员会(17ZR1429900)
上海市化学生物学重点实验室开放基金资助
C—H Cyanoalkylation:the Direct C—H Cyanomethylation of Naphthalimide
Received date: 2020-10-09
Revised date: 2021-01-14
Online published: 2021-03-04
Supported by
Natural Science Foundation of Shanghai(17ZR1429900)
Opening Fund of Shanghai Key Laboratory of Chemical Biology.
陈曦 , 李喆垚 , 陈亚运 , 陈志华 , 胡艳 , 刘传祥 . C—H氰烷基化:导向基控制的萘酰亚胺C—H氰烷基化[J]. 化学进展, 2021 , 33(11) : 1947 -1952 . DOI: 10.7536/PC201001
The cyanoalkylation/cyanomethylation of organic molecules is of great research interest to organic and medicinal chemists due to the wide presence of the cyano group in biologically active molecules and the facile conversion of the cyano group into many other functional groups, such as amides, esters, aldehydes, and primary amines. Although a variety of different synthetic strategies have been developed for the selective introduction of the cyanomethyl group, an attractive approach is to use acetonitrile directly through C—H activation due to the highly efficient atom economy and the avoidance of prefunctionalization. Therefore, this review summarizes the main research progress in C—H cyanoalkylation/cyanomethylation of radical cyanomethylation, Photochemical Cross-coupling reaction, Cross-Dehydrogenative Coupling(CDC) Reaction, Directing group-promoted C—H cyanomethylation and Fluorophore C—H cyanomethylation reported by our group.
Contents
1 Introduction
2 Radical cyanomethylation of activated alkenes with acetonitrile
3 Photochemical cross-coupling reaction of alkene or aroma C(sp2)—H functionalization of acetonitrile
4 Cross-dehydrogenative coupling(CDC) reaction of aromatic ring C(sp2)—H functionalization of acetonitrile
5 Directing group-promoted C—H cyanomethylation
6 Conclusion and outlook

Key words: cyanomethylation; C—H functionalization; radical; directing group; naphthalimide




















| [1] |
Mᶏkosza M. Chem. Soc. Rev., 2010, 39(8): 2855.
|
| [2] |
Rappoport Z.[M]. Chichester, UK: John Wiley & Sons, Ltd., 1970.
|
| [3] |
Dénès F, Pérez-Luna A, Chemla F. Chem. Rev., 2010, 110(4): 2366.
|
| [4] |
Weiberth F J, Hall S S. J. Org. Chem., 1987, 52(17): 3901.
|
| [5] |
Trivedi B K, Holmes A, Stoeber T L, Blankley C J, Roark W H, Picard J A, Shaw M K, Essenburg A D, Stanfield R L, Krause B R. J. Med. Chem., 1993, 36(22): 3300.
|
| [6] |
Culkin D A, Hartwig J F. Acc. Chem. Res., 2003, 36(4): 234.
|
| [7] |
Chen G, Wang Z, Wu J, Ding K L. Org. Lett., 2008, 10(20): 4573.
|
| [8] |
Narsaiah A, Nagaiah K. Adv. Synth. Catal., 2004, 346(11): 1271.
|
| [9] |
Bacchi S, Stazi F, Maton W, Castoldi D, Westerduin P, Curcuruto O. Synthesis, 2010, 2010(19): 3332.
|
| [10] |
Yang Y Y, Tang S, Liu C, Zhang H M, Sun Z X, Lei A W. Org. Biomol. Chem., 2011, 9(15): 5343.
|
| [11] |
Wu L Y, Hartwig J F. J. Am. Chem. Soc., 2005, 127(45): 15824.
|
| [12] |
Shang R, Ji D S, Chu L, Fu Y, Liu L. Angew. Chem. Int. Ed., 2011, 50(19): 4470.
|
| [13] |
Lovato K, Guo L R, Xu Q L, Liu F T, Yousufuddin M, Ess D H, Kürti L, Gao H Y. Chem. Sci., 2018, 9(41): 7992.
|
| [14] |
Dong Z, Wang J C, Dong G B. J. Am. Chem. Soc., 2015, 137(18): 5887.
|
| [15] |
Cornella J, Righi M, Larrosa I. Angew. Chem. Int. Ed., 2011, 50(40): 9429.
|
| [16] |
Wu T, Mu X, Liu G S. Angew. Chem. Int. Ed., 2011, 50(52): 12578.
|
| [17] |
Li J, Wang Z G, Wu N J, Gao G, You J S. Chem. Commun., 2014, 50(95): 15049.
|
| [18] |
Li Z J, Xiao Y X, Liu Z Q. Chem. Commun., 2015, 51(49): 9969.
|
| [19] |
Pan C D, Zhang H L, Zhu C J. Org. Biomol. Chem., 2015, 13(2): 361.
|
| [20] |
Bunescu A, Wang Q, Zhu J P. Org. Lett., 2015, 17(8): 1890.
|
| [21] |
Chatalova-Sazepin C, Wang Q, Sammis G M, Zhu J P. Angew. Chem. Int. Ed., 2015, 54(18): 5443.
|
| [22] |
Liu Y Y, Yang X H, Song R J, Luo S L, Li J H. Nat. Commun., 2017, 8: 14720.
|
| [23] |
Wang K C, Chen X, Yuan M, Yao M, Zhu H C, Xue Y B, Luo Z W, Zhang Y H. J. Org. Chem., 2018, 83(3): 1525.
|
| [24] |
Yoshida H, Fujimura Y, Yuzawa H, Kumagai J, Yoshida T. Chem. Commun., 2013, 49(36): 3793.
|
| [25] |
Zhang J L, Liu Y, Song R J, Jiang G F, Li J H. Synlett., 2014, 25: 1031.
|
| [26] |
Wada E, Takeuchi T, Fujimura Y, Tyagi A, Kato T, Yoshida H. Catal. Sci. Technol., 2017, 7(12): 2457.
|
| [27] |
Liu Z Q, Li Z J. Chem. Commun., 2016, 52(99): 14278.
|
| [28] |
Su H M, Wang L Y, Rao H H, Xu H. Org. Lett., 2017, 19(9): 2226.
|
| [29] |
Zhang R X, Jin S Z, Liu Q, Lin S, Yan Z H. J. Org. Chem., 2018, 83(21): 13030.
|
| [30] |
Liu Y B, Yang K, Ge H B. Chem. Sci., 2016, 7(4): 2804.
|
| [31] |
Qiao K, Zhang D, Zhang K, Yuan X, Zheng M W, Guo T F, Fang Z, Wan L, Guo K. Org. Chem. Front., 2018, 5(7): 1129.
|
| [32] |
Davis R B, Pizzini L C. J. Org. Chem., 1960, 25(11): 1884.
|
| [33] |
Mᶏkosza M, Wojciechowski K. Chem. Rev., 2004, 104(5): 2631.
|
| [34] |
Mᶏkosza M, Staliński K. Tetrahedron, 1998, 54(30): 8797.
|
| [35] |
Chen J J, Liu C X, Zhang J L, Ding W, Zhou M, Wu F H. Chem. Commun., 2013, 49(92): 10814.
|
| [36] |
Wu H W, Chen Y Y, Rao C H, Liu C X. Prog. Chem., 2016, 28(10): 1501.
(伍宏伟, 陈亚运, 饶才辉, 刘传祥. 化学进展, 2016, 28(10): 1501).
|
| [37] |
Duke R M, Veale E B, Pfeffer F M, Kruger P E, Gunnlaugsson T. Chem. Soc. Rev., 2010, 39(10): 3936.
|
| [38] |
Zhou L, Xie L J, Liu C H, Xiao Y. Chin. Chem. Lett., 2019, 30(10): 1799.
|
| [39] |
McDonald K P, Ramabhadran R O, Lee S, Raghavachari K, Flood A H. Org. Lett., 2011, 13(23): 6260.
|
| [40] |
Xu Z C, Xiao Y, Qian X H, Cui J N, Cui D W. Org. Lett., 2005, 7(5): 889.
|
| [41] |
Chen Y Y, Hu X X, Rao C H, Li Z Y, Chen L, Fu C, Liu C X. Anal., 2018, 143(19): 4655.
|
| [42] |
Li Z Y, Rao C H, Chen L, Fu C, Zhu T T, Chen X, Liu C X. J. Org. Chem., 2019, 84(11): 7518.
|
| [43] |
Li Z Y, Rao C H, Chen L, Fu C, Zhu T T, Chen X, Liu C X. Dyes Pigments, 2020, 173: 107967.
|
/
| 〈 |
|
〉 |