1 引言
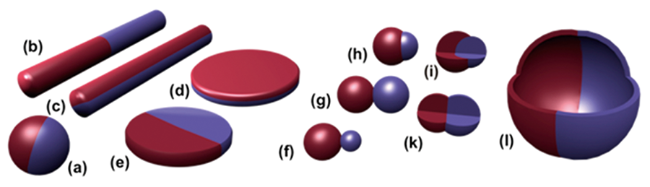
图1 Janus粒子的类型(球形(a),圆柱形(b,c),圆盘形(d,e),不对称或雪人形(f), 对称哑铃型(g,k),相嵌型(h),反常型(i),囊泡或胶囊型(l))[30]Fig. 1 Different types of Janus particles(spherical(a), cylindrical(b, c), disc-shaped(d, e), asymmetric or snowman(f), symmetric appearance(g, k), attached nodes(h), eccentric encapsulation(i), and vesicles or capsules(l))[30] |
2 两亲性Janus粒子的制备方法
2.1 表面改性法制备两亲性Janus粒子
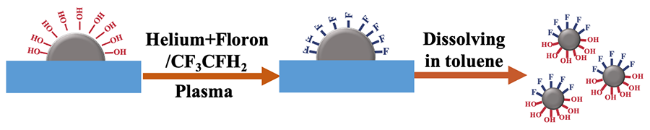
2.1.1 半屏蔽法
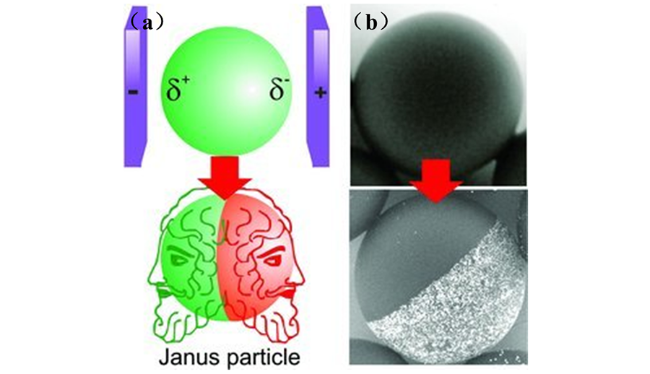
2.1.2 两相界面法
图3 (a)固-液界面处两亲性SiO2 Janus粒子的合成示意图;(b)用金纳米颗粒标记的Janus粒子的TEM图像,SiO2纳米颗粒一面被金纳米颗粒标记,另一面未被标记;(c)用金纳米颗粒标记的氨基化SiO2粒子的TEM图像,不同于Janus颗粒,SiO2粒子所有表面均被标记[49]Fig.3 (a) Schematic synthesis of Janus silica nanoparticles at the interface of a Pickering emulsion.(b) TEM images of Janus nanoparticles labeled with Au nanoparticles; one side of the SiO2 nanoparticles is labeled with Au nanoparticles, whereas the other side is not labeled.(c) TEM image of amino-modified SiO2 nanoparticles labeled with Au nanoparticles. Different from the Janus nanoparticles, all of the sides were labeled[49] |
2.1.3 电化学沉积法
图4 电化学沉积法制备Janus粒子:(a)双极电化学原理示意图;(b)双极电沉积前后玻璃碳颗粒的SEM图像(粒径为约20 μm) [50]Fig.4 The synthesis of Janus particles by bipolar electrodeposition:(a) Principle of bipolar electrochemistry;(b) SEM images of glassy carbon particles before and after bipolar electrodeposition.(The particle diameter is around 20 μm) [50] |
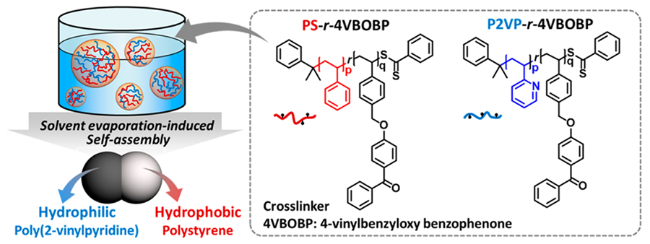
2.2 原位生成法制备两亲性Janus粒子
2.2.1 微流体法
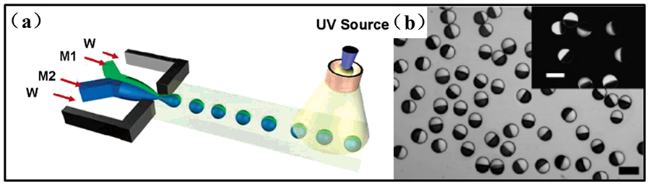
图5 (a)微流体装置合成两亲性Janus粒子的示意图;(b)两亲性Janus粒子的光学显微镜图像(亮相和暗相分别是M1和M2的聚合物,比例尺为100 μm) [55]Fig.5 (a) Schematic diagram of synthesis of amphiphilic Janus particles by microfluidic device;(b) Optical microscope image of amphiphilic Janus particles(The bright and dark phase of polymer are M1 and M2, respectively, scale of 100 μm) [55] |
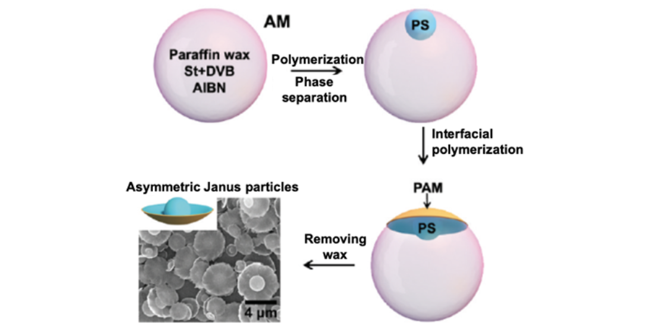
2.2.2 相分离法
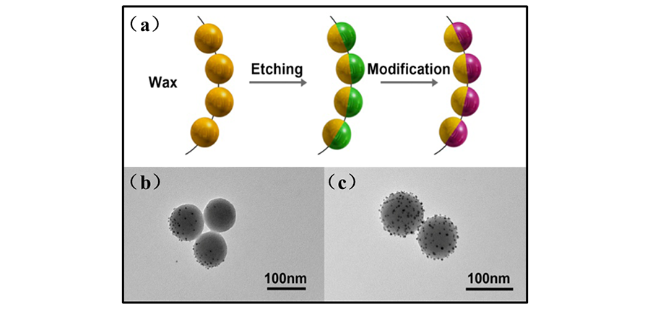
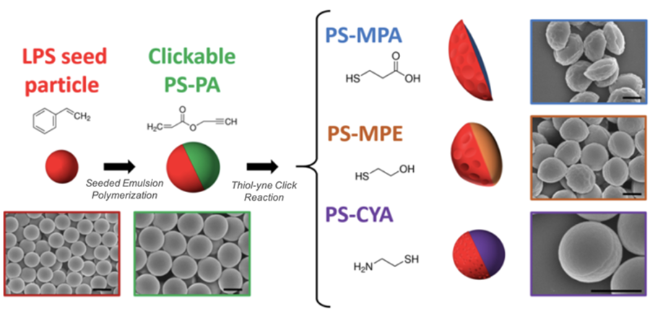
2.2.3 点击化学法
表1 两亲性Janus粒子不同制备方法的优缺点Table 1 Advantages and disadvantages of different preparation methods of amphiphilic Janus particles |
| Preparation | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Protecting mask techniques | Regular morphology | Low yield | 2,39 |
| Two-phase interface method | Mature technology, simple method, large range of particle size | Difficulty in separation, low yield | 45,47,48 |
| Electrochemical deposition | No mask required | Only for conductive material | 2,50 |
| Microfluidic method | Controllable size, good monodispersity | Large particle size and slow synthesis process | 2,54,58 |
| Phase separation | Good universality, small size, easy to scale up production | Poor morphology regularity, only for organic materials | 21,61 |
| Click chemistry | Strictly controllable morphology and chemical composition, fast reaction speed and high efficiency | Aiming at materials with specific functional groups | 64,65 |
3 两亲性Janus粒子在Pickering乳液中的研究进展
3.1 两亲性Janus粒子亲疏水平衡性对Pickering乳液稳定性的影响
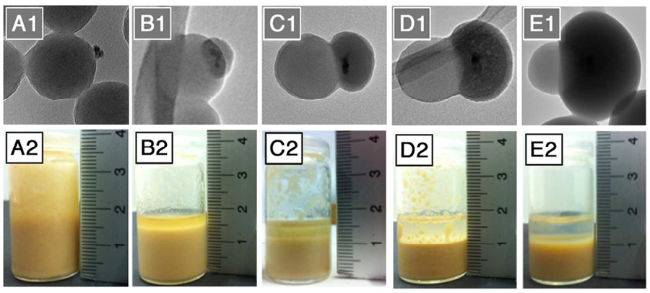
图9 (A1~E1)Janus粒子的透射电镜照片(二氧化硅半球尺寸从A1到E1分别为0、50、90、110和200 nm);(A2-E2)相应Janus粒子稳定甲苯-水乳液的外观照片[80]Fig. 9 (A1~E1) Transmission electron microscopy photos of Janus particles(The size of silica hemisphere from A1 to E1 are 0, 50, 90, 110 and 200 nm);(A2~E2) The corresponding photos of toluene-water emulsion stabilized by Janus particles[80] |
3.2 两亲性Janus粒子形状对Pickering乳液稳定性的影响
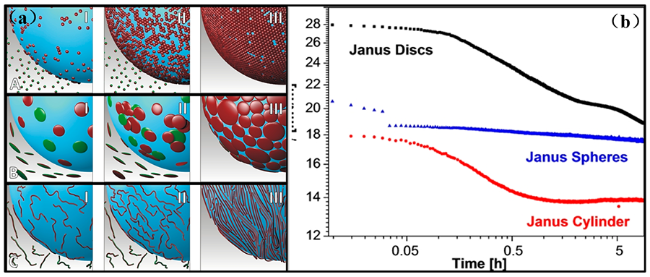
图10 (a)不同形状Janus粒子的吸附行为:(A)球形Janus;(B)圆盘形Janus;(C)圆柱形Janus;(b)不同形状Janus粒子对水/甲苯界面张力的影响[74]Fig. 10 (a)The most typical adsorption stages pointed out for(A) Janus spheres,(B) Janus discs, and(C) Janus cylinders;(b)The influence of the Janus particle shape on the interfacial tensionata water/toluene interface[74] |