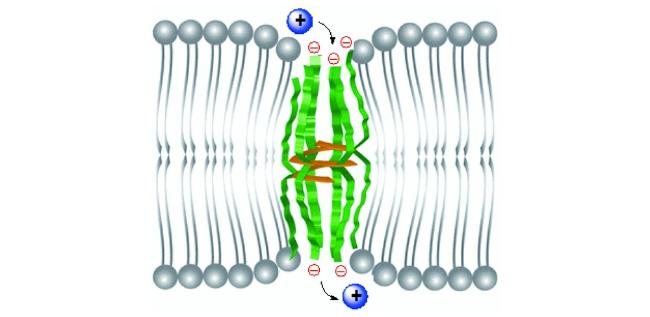
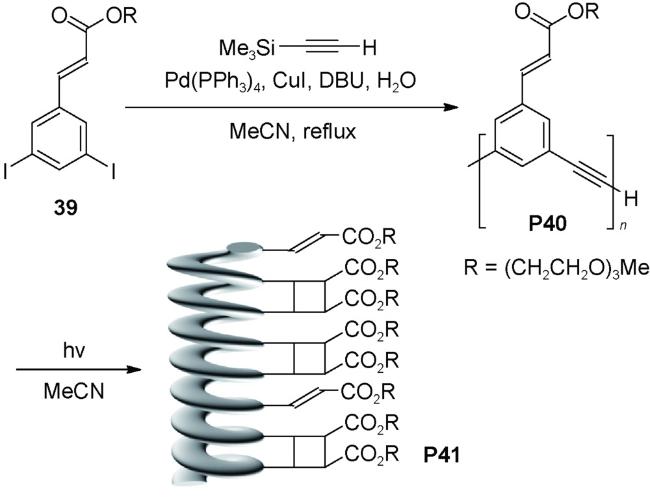
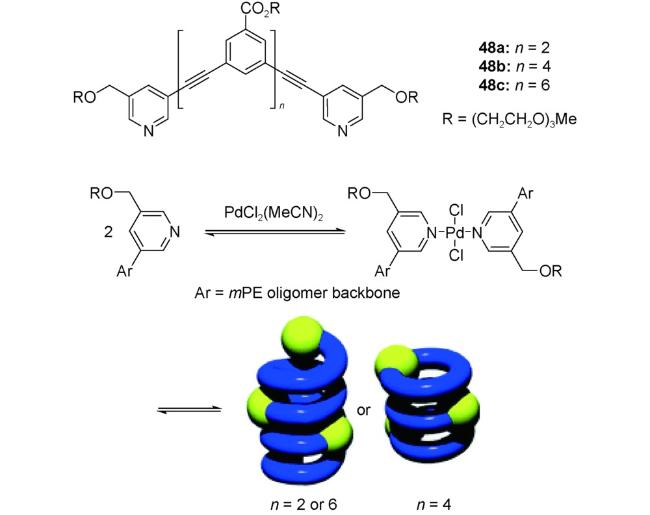
1 引言
2 芳香寡聚螺旋管
2.1 芳香酰胺和酰肼构筑基元
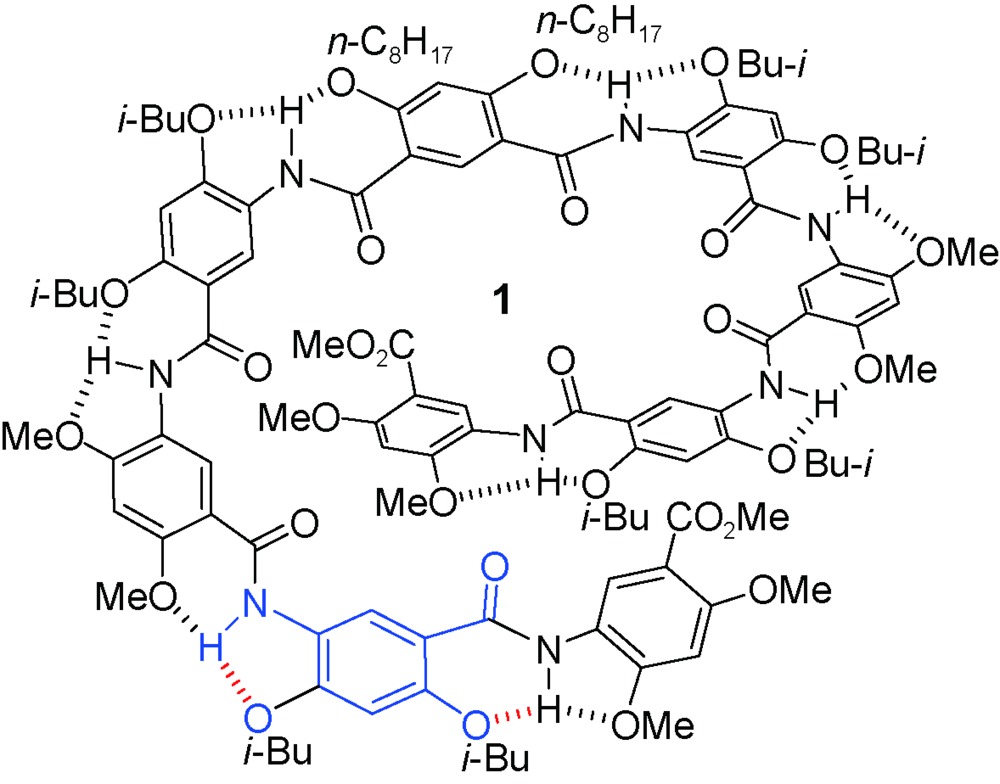
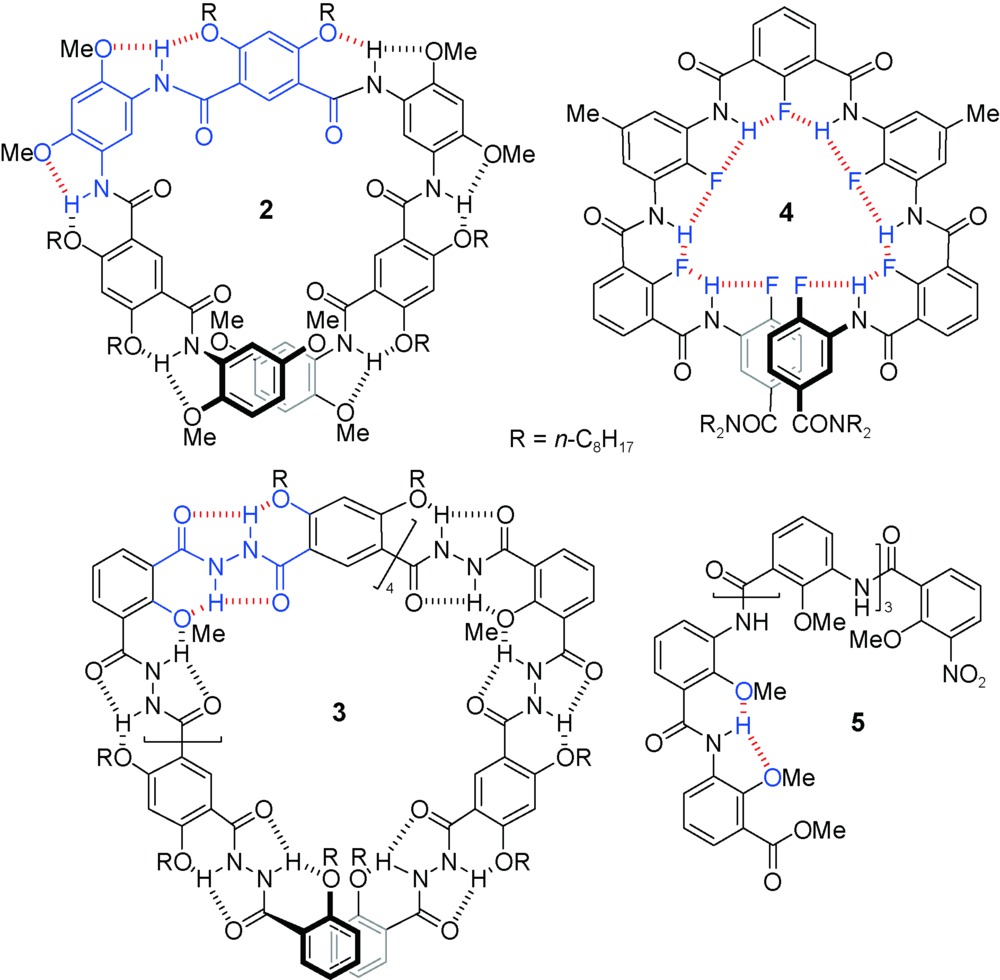
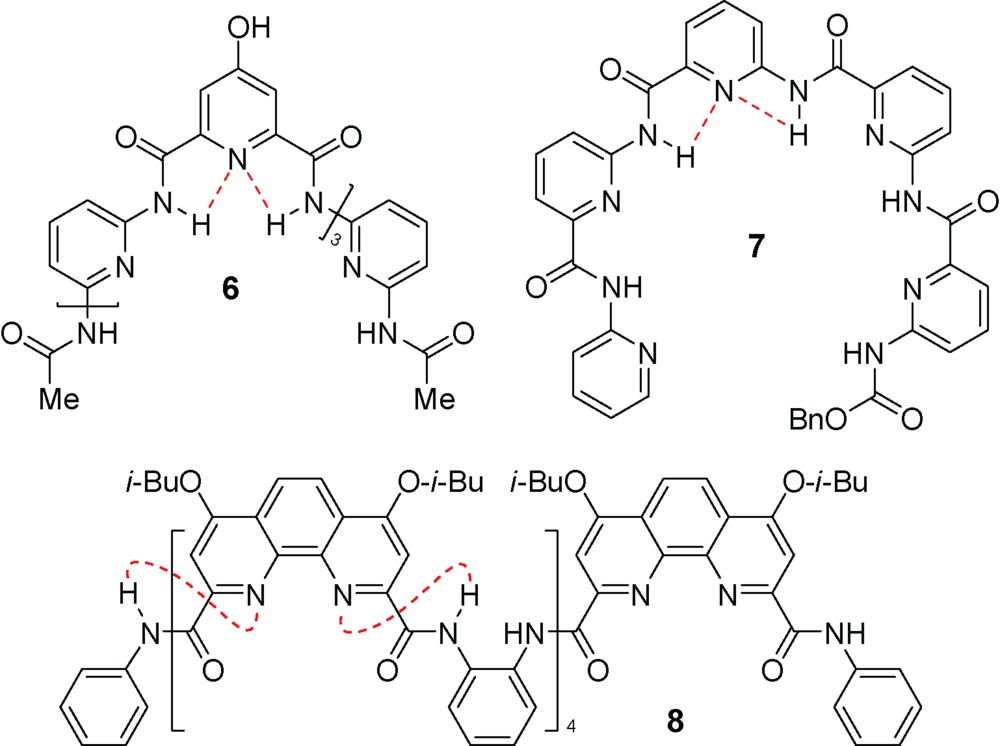
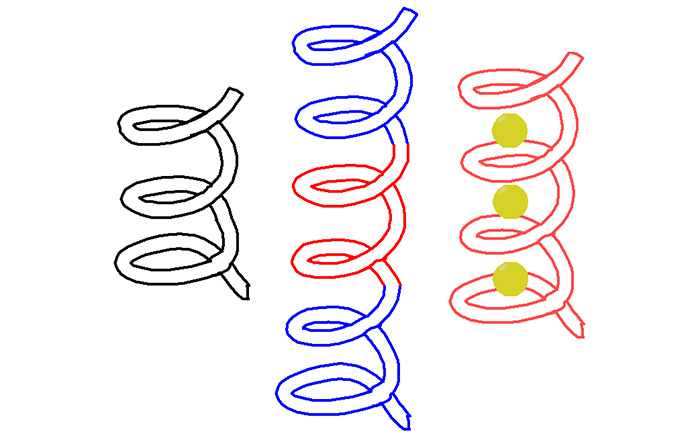
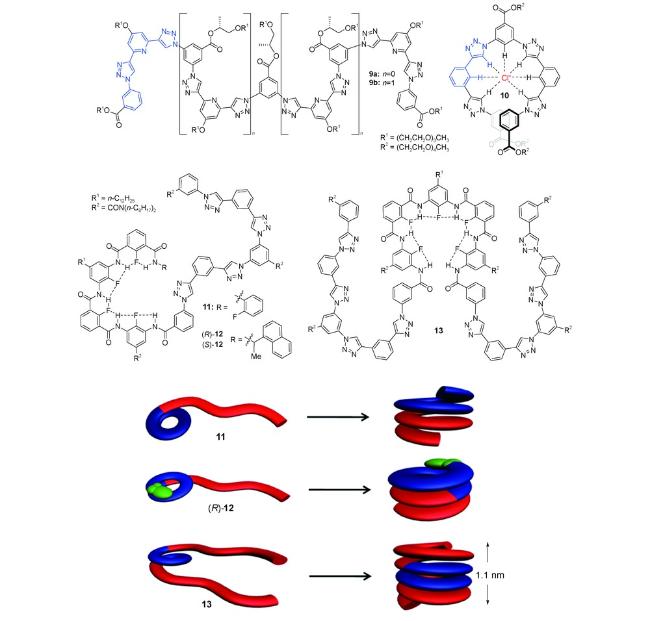
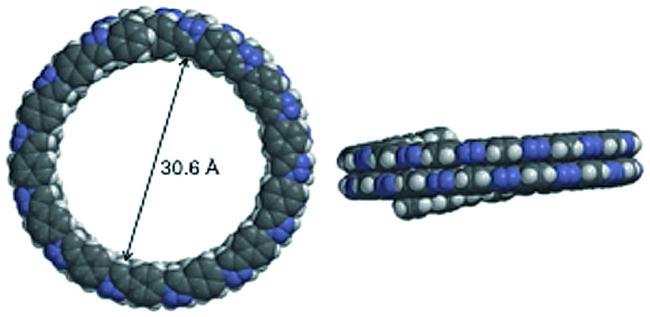
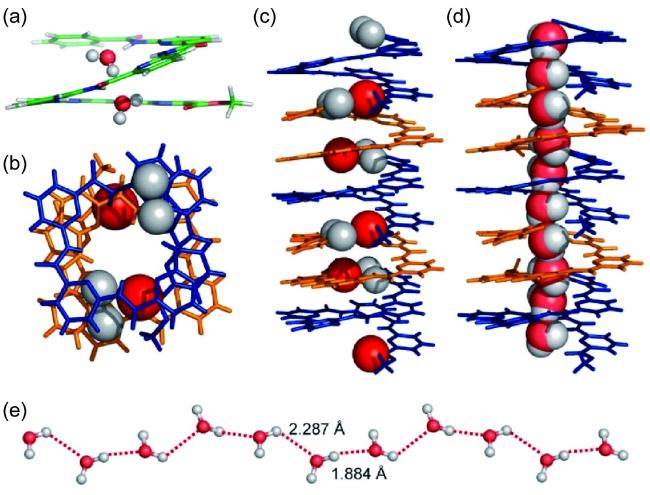
 C)氢键将烷基化的手性单糖、双糖等稳定在螺旋体空腔[28,29],手性单糖还进一步诱导产生了手性螺旋。寡聚体3有13个重复单元,能够形成约2.5圈的螺旋构象,较深的内穴使其与单糖的结合作用也比较强。较短的寡聚体4和5所形成的内穴则比较浅,并且控制分子构象的分子内氢键在空腔内部,内穴尺寸较小,然而这两个寡聚体仍能够作为主体分子结合铵盐[30,31]。寡聚螺旋管的内穴深度由于芳香砌块重复单元数量的不同而不同。8-氨基喹啉-2-甲酸三聚体能够形成一圈螺旋,其34-聚体形成了长度达到5.1 nm的螺旋管[32]。在其N-端和C-端分别引入电子给体和电子受体实现了光诱导的电子转移。吡啶寡聚体7的晶体结构(图1)表明约4.3个重复单元即可以形成一圈螺旋,但其空腔较小,仅能容纳水分子[33,34]。引入邻二氮杂菲的寡聚体8没有形成酰胺基团参与的分子内氢键,而是与吡啶的氮原子形成了分子内五元环N—H…N氢键,通过这种分子内氢键作用使整个分子发生折叠,形成了一个没有可利用空腔的螺旋结构。晶体结构显示(图2),所有酰胺的羰基氧原子朝向螺旋的外部,水分子通过分子间氢键作用占据了螺旋体的空腔[35]。吡啶、喹啉、吡啶并喹啉等芳杂环酰胺单元通过合理的序列设计形成螺旋结构,这些螺旋体与其他分子可以组装形成分子梭等主客体超分子结构[36,37,38]。2,6-二氨基吡啶与2,6-吡啶二酸的缩合寡聚体、7-氨基8-氟-2-喹啉酸的寡聚体等也形成螺旋结构,并进一步组装为双螺旋或多重螺旋结构,但这些螺旋结构的空腔尺寸较小,无法容纳客体分子[26,39,40]。
C)氢键将烷基化的手性单糖、双糖等稳定在螺旋体空腔[28,29],手性单糖还进一步诱导产生了手性螺旋。寡聚体3有13个重复单元,能够形成约2.5圈的螺旋构象,较深的内穴使其与单糖的结合作用也比较强。较短的寡聚体4和5所形成的内穴则比较浅,并且控制分子构象的分子内氢键在空腔内部,内穴尺寸较小,然而这两个寡聚体仍能够作为主体分子结合铵盐[30,31]。寡聚螺旋管的内穴深度由于芳香砌块重复单元数量的不同而不同。8-氨基喹啉-2-甲酸三聚体能够形成一圈螺旋,其34-聚体形成了长度达到5.1 nm的螺旋管[32]。在其N-端和C-端分别引入电子给体和电子受体实现了光诱导的电子转移。吡啶寡聚体7的晶体结构(图1)表明约4.3个重复单元即可以形成一圈螺旋,但其空腔较小,仅能容纳水分子[33,34]。引入邻二氮杂菲的寡聚体8没有形成酰胺基团参与的分子内氢键,而是与吡啶的氮原子形成了分子内五元环N—H…N氢键,通过这种分子内氢键作用使整个分子发生折叠,形成了一个没有可利用空腔的螺旋结构。晶体结构显示(图2),所有酰胺的羰基氧原子朝向螺旋的外部,水分子通过分子间氢键作用占据了螺旋体的空腔[35]。吡啶、喹啉、吡啶并喹啉等芳杂环酰胺单元通过合理的序列设计形成螺旋结构,这些螺旋体与其他分子可以组装形成分子梭等主客体超分子结构[36,37,38]。2,6-二氨基吡啶与2,6-吡啶二酸的缩合寡聚体、7-氨基8-氟-2-喹啉酸的寡聚体等也形成螺旋结构,并进一步组装为双螺旋或多重螺旋结构,但这些螺旋结构的空腔尺寸较小,无法容纳客体分子[26,39,40]。2.2 芳香三氮唑构筑基元

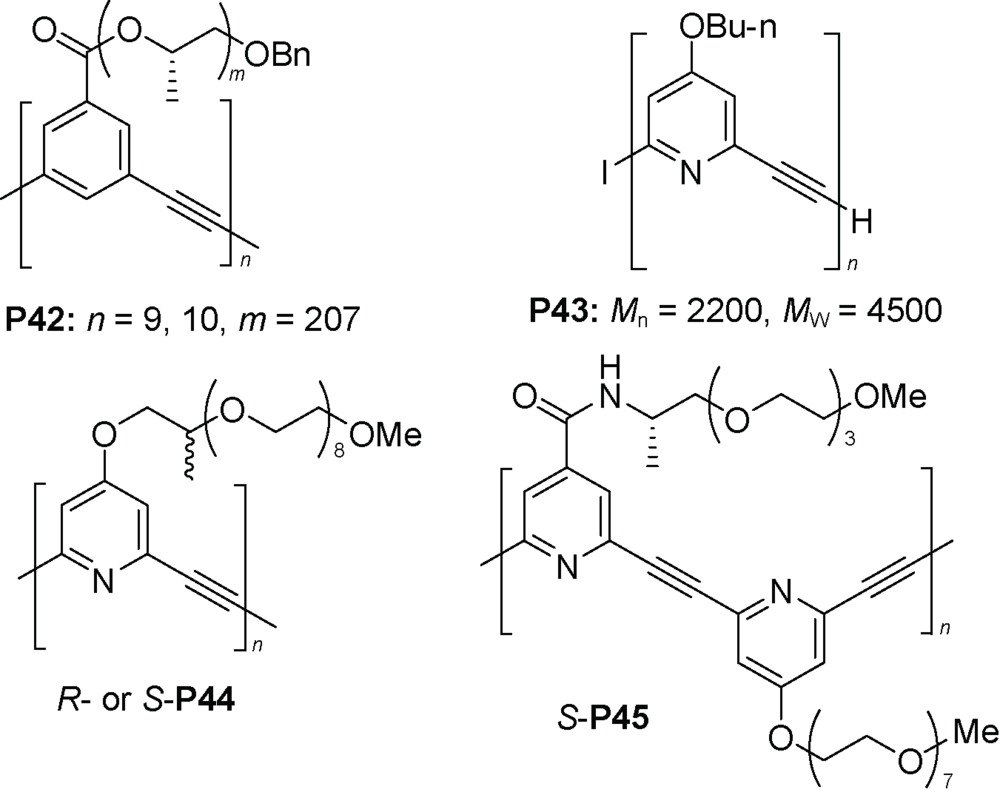
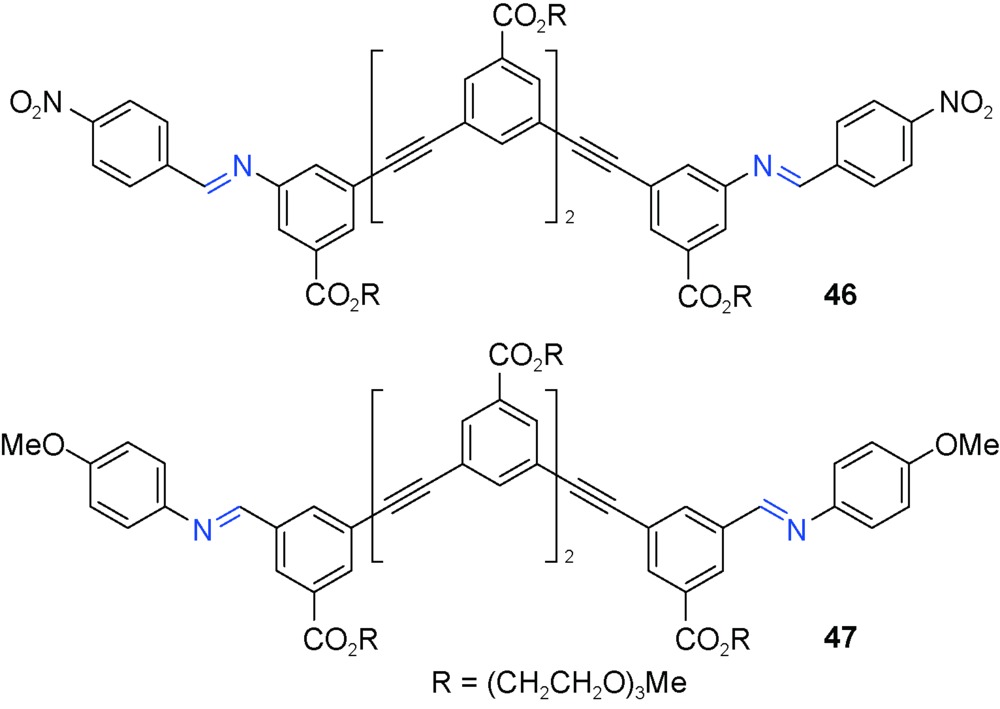
2.3 间-苯乙炔和杂环类似物构筑基元
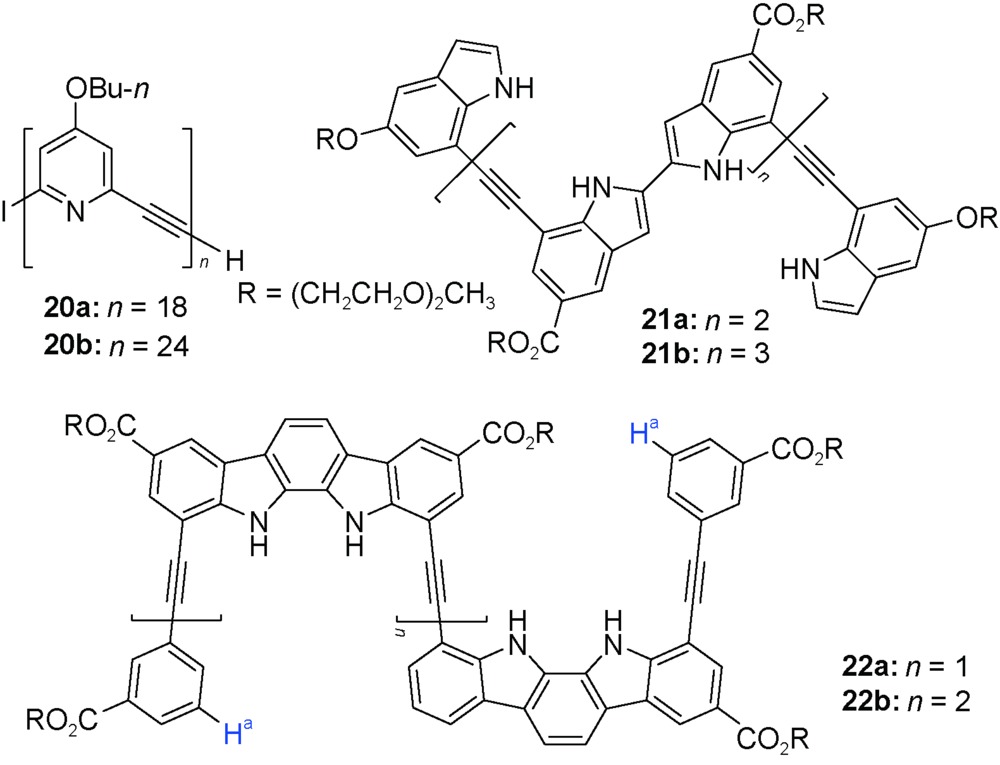
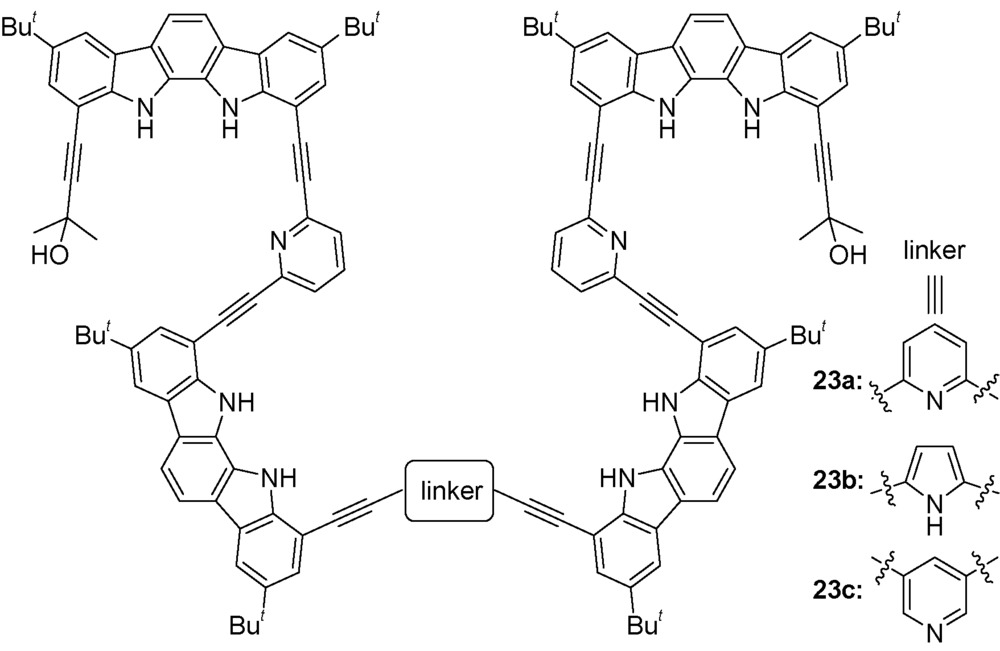
3 芳香聚合物螺旋管
3.1 芳香酰胺和类似物构筑基元
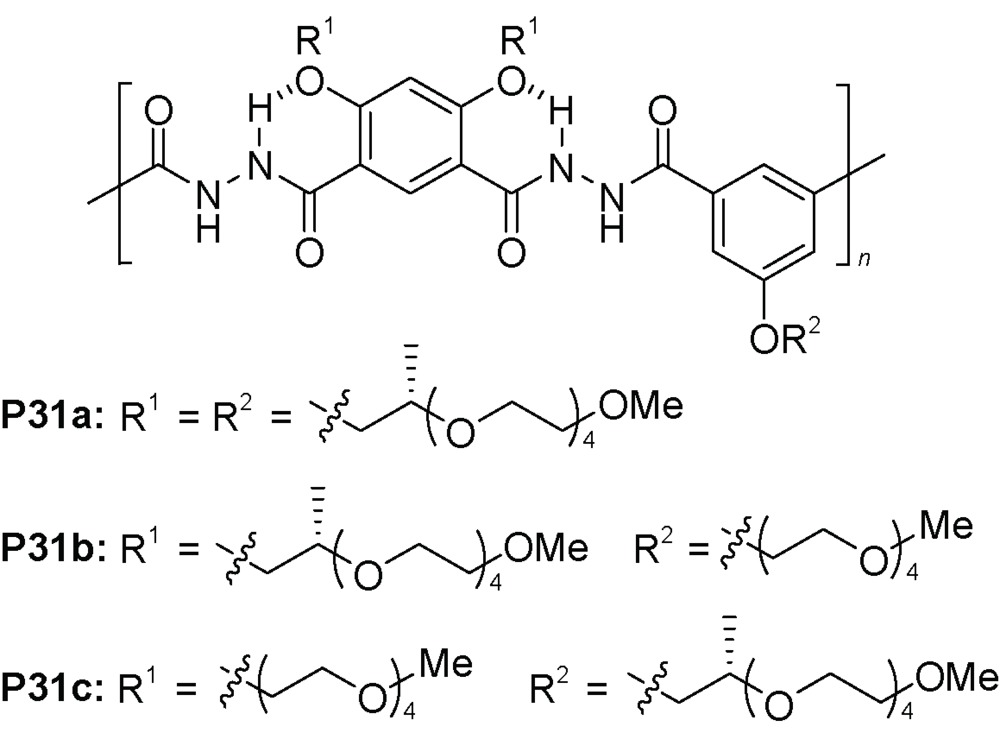
 C) 氢键则进一步稳定了螺旋构象。圆二色谱实验发现,随着链长逐渐增加,Cotton效应不断增强,说明侧链的手性会诱导螺旋管产生手性,并且具有协同效应。
C) 氢键则进一步稳定了螺旋构象。圆二色谱实验发现,随着链长逐渐增加,Cotton效应不断增强,说明侧链的手性会诱导螺旋管产生手性,并且具有协同效应。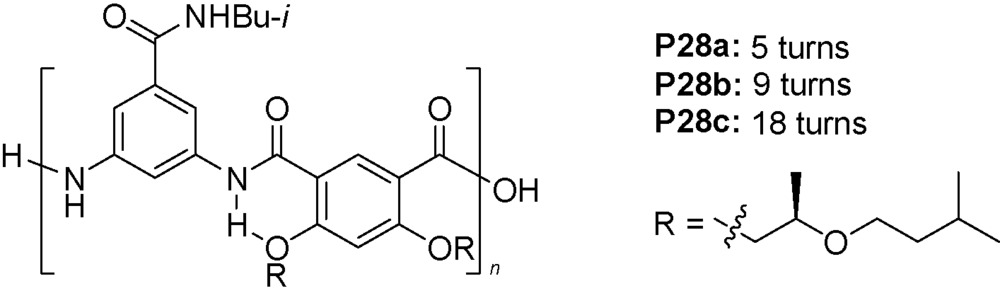
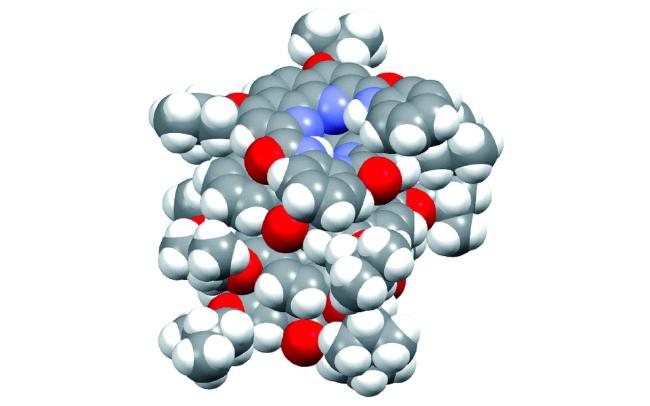
 C)氢键作用发生二聚形成同源双链[70]。对两亲性聚合物P29的研究进一步展现了不存在分子内氢键的聚芳酰胺在溶液中的折叠行为[71]。聚合物P29a的数均分子量Mn和重均分子量Mw分别为32.0和59.0 kDa(PDI=1.8)。聚合物P29a在水溶液及DMF、MeOH、CHCl3、CH2Cl2等多种不同极性的有机溶剂中均形成了螺旋结构(图4)。对聚合物P29a进行结构模拟可见,引入的较大尺寸芳香萘二胺单元不仅提供了更好的层间芳香堆积,还调控骨架折叠形成了1.3 nm的较大内径,螺旋结构的螺距为3.5 Å,其形成了9圈螺旋,因此螺旋管的深度达到3.1 nm。聚合物P29a的酰胺氮原子上甲基化得到P29b。P29a和P29b在不同极性溶剂中表现不同折叠行为,在低极性的二氯甲烷、氯仿等有机溶剂中,甲基化的P29b形成螺旋构象的倾向大大降低,说明除了疏溶剂作用,螺旋层间的分子内N—H…O(
C)氢键作用发生二聚形成同源双链[70]。对两亲性聚合物P29的研究进一步展现了不存在分子内氢键的聚芳酰胺在溶液中的折叠行为[71]。聚合物P29a的数均分子量Mn和重均分子量Mw分别为32.0和59.0 kDa(PDI=1.8)。聚合物P29a在水溶液及DMF、MeOH、CHCl3、CH2Cl2等多种不同极性的有机溶剂中均形成了螺旋结构(图4)。对聚合物P29a进行结构模拟可见,引入的较大尺寸芳香萘二胺单元不仅提供了更好的层间芳香堆积,还调控骨架折叠形成了1.3 nm的较大内径,螺旋结构的螺距为3.5 Å,其形成了9圈螺旋,因此螺旋管的深度达到3.1 nm。聚合物P29a的酰胺氮原子上甲基化得到P29b。P29a和P29b在不同极性溶剂中表现不同折叠行为,在低极性的二氯甲烷、氯仿等有机溶剂中,甲基化的P29b形成螺旋构象的倾向大大降低,说明除了疏溶剂作用,螺旋层间的分子内N—H…O( C)氢键是稳定螺旋构象的决定因素。在甲醇等大极性溶剂中,疏溶剂作用是形成螺旋结构的决定因素,这时聚合物P29a和P29b有类似的折叠行为。萘二胺单元的1,8-酰亚胺基团对聚合物形成螺旋结构也起到重要作用,没有1,8-酰亚胺基团时,聚合物不能自行折叠形成螺旋结构。在氯仿中聚合物与l-天冬氨酸等手性氨基酸二价盐通过多价分子间氢键诱导产生手性螺旋结构[72]。70% ee值的天冬氨酸负离子就能诱导聚合物产生最强Cotton效应,说明在螺旋形成过程中遵循多数决原则。螺旋的手性还与溶剂有很大关系,在氯仿中逐渐加入乙腈将改变螺旋结构的手性,在乙腈溶液中产生与氯仿溶剂中完全相反的手性。由间-苯二胺与2,2'-联吡啶单元交替偶联形成的聚芳酰胺在水溶液中也能形成螺旋结构[73]。苯基侧链的缬氨酸单元将手性传递给螺旋结构。由于联吡啶与Ni2+配位而改变构象,从而产生了手性相反的2种螺旋结构。
C)氢键是稳定螺旋构象的决定因素。在甲醇等大极性溶剂中,疏溶剂作用是形成螺旋结构的决定因素,这时聚合物P29a和P29b有类似的折叠行为。萘二胺单元的1,8-酰亚胺基团对聚合物形成螺旋结构也起到重要作用,没有1,8-酰亚胺基团时,聚合物不能自行折叠形成螺旋结构。在氯仿中聚合物与l-天冬氨酸等手性氨基酸二价盐通过多价分子间氢键诱导产生手性螺旋结构[72]。70% ee值的天冬氨酸负离子就能诱导聚合物产生最强Cotton效应,说明在螺旋形成过程中遵循多数决原则。螺旋的手性还与溶剂有很大关系,在氯仿中逐渐加入乙腈将改变螺旋结构的手性,在乙腈溶液中产生与氯仿溶剂中完全相反的手性。由间-苯二胺与2,2'-联吡啶单元交替偶联形成的聚芳酰胺在水溶液中也能形成螺旋结构[73]。苯基侧链的缬氨酸单元将手性传递给螺旋结构。由于联吡啶与Ni2+配位而改变构象,从而产生了手性相反的2种螺旋结构。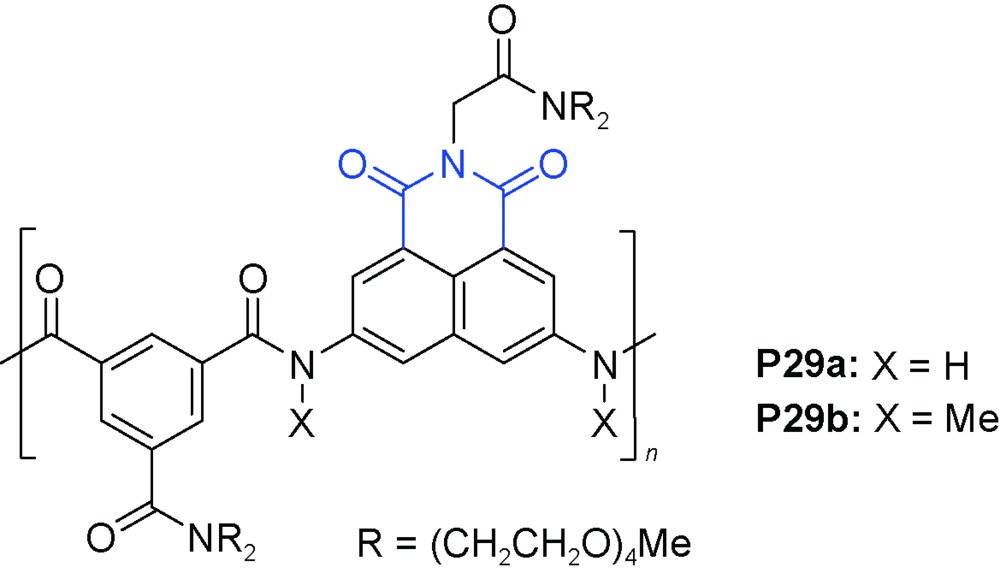
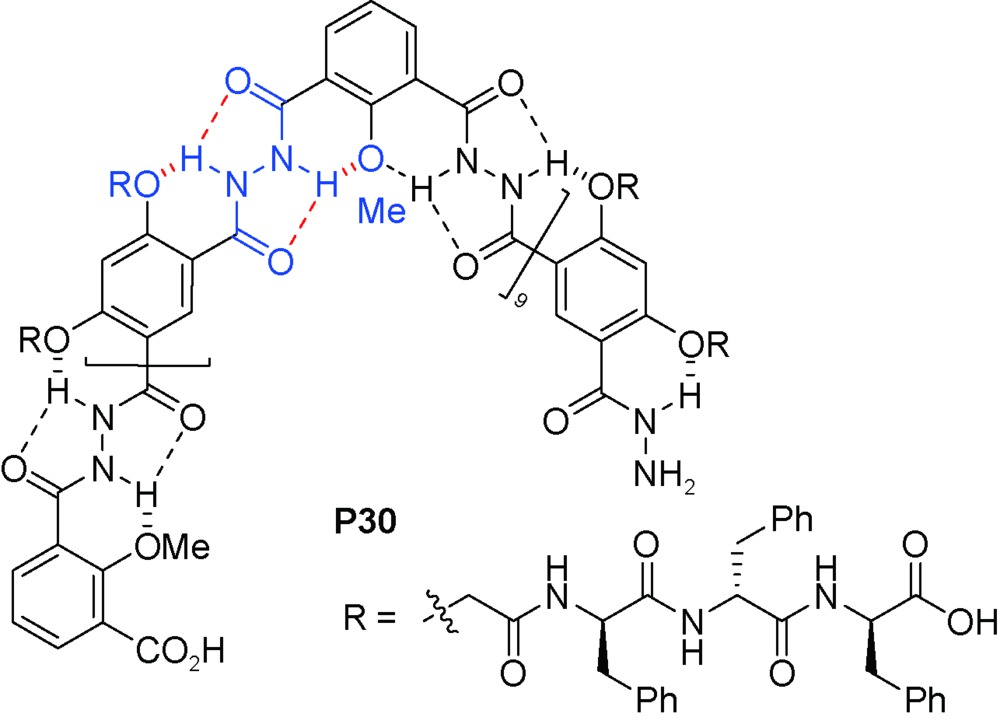
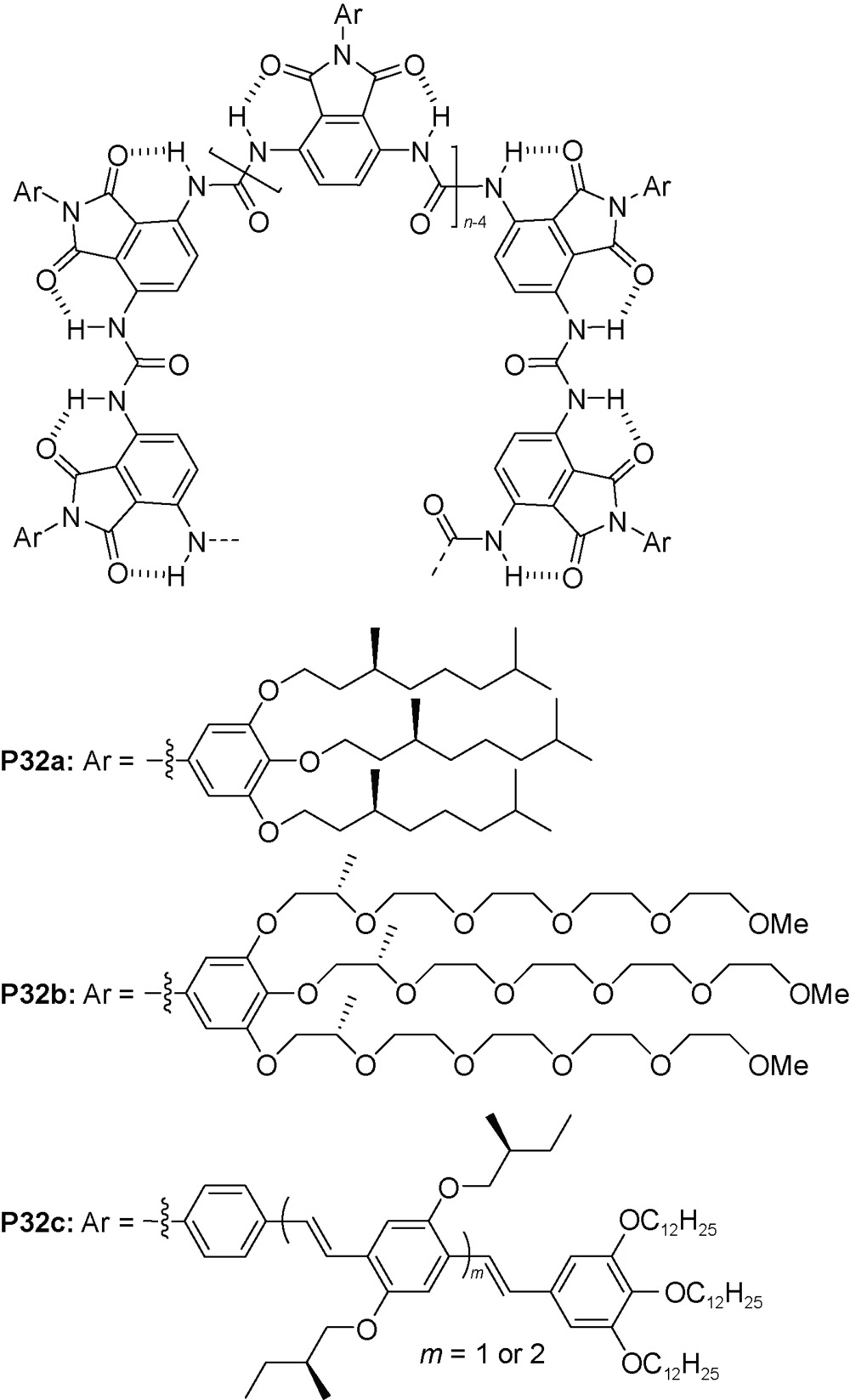
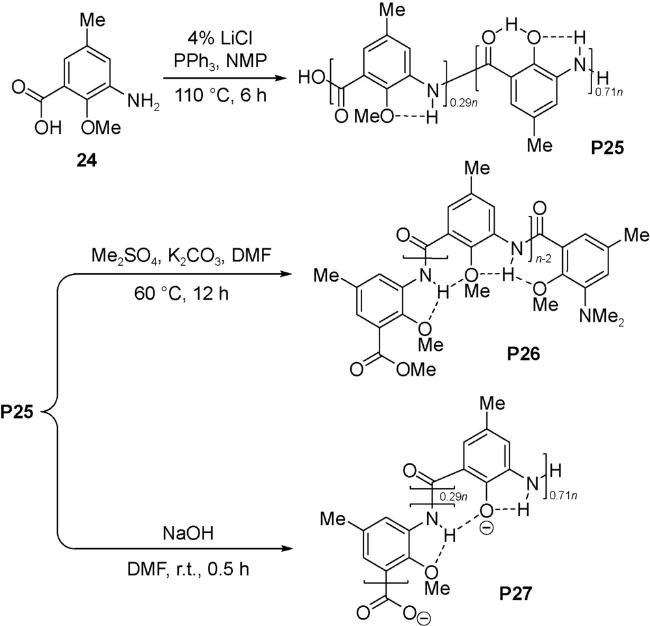
 C) 氢键使聚合物有刚性结构。聚合度较高的聚合物P32a(n=~30) 在THF中形成手性螺旋[77]。尿素单元形成顺式构象,每6~8个重复单元形成一圈螺旋,所以聚合物P32a约形成4~5圈螺旋,层间芳香堆积作用是螺旋构象的重要稳定因素。而在CHCl3溶剂中,由于分子内N—H…O(
C) 氢键使聚合物有刚性结构。聚合度较高的聚合物P32a(n=~30) 在THF中形成手性螺旋[77]。尿素单元形成顺式构象,每6~8个重复单元形成一圈螺旋,所以聚合物P32a约形成4~5圈螺旋,层间芳香堆积作用是螺旋构象的重要稳定因素。而在CHCl3溶剂中,由于分子内N—H…O( C) 氢键被破坏,聚合物P32a不能形成螺旋结构。
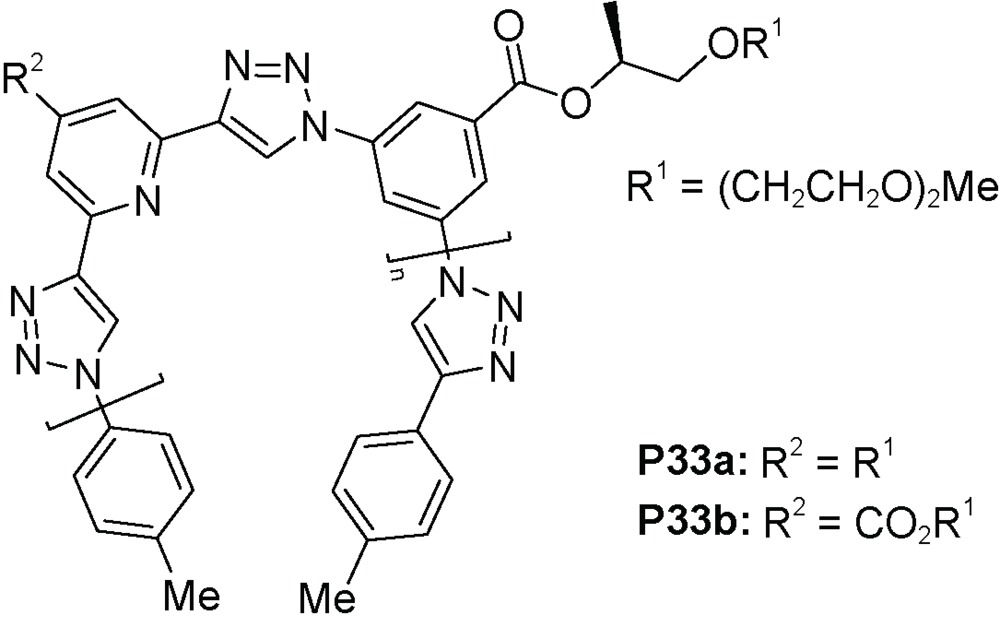
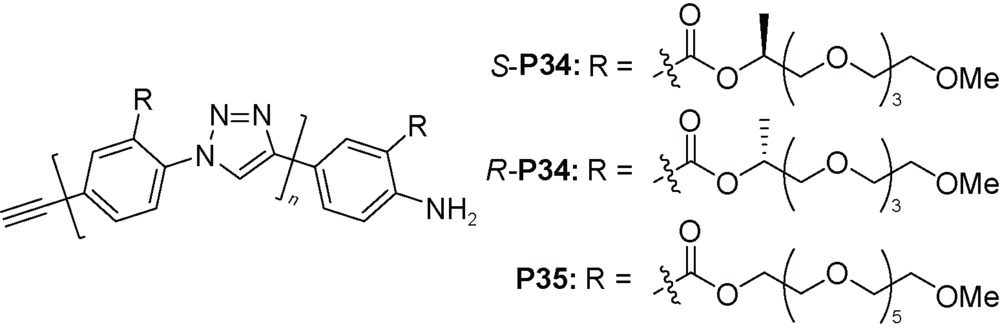
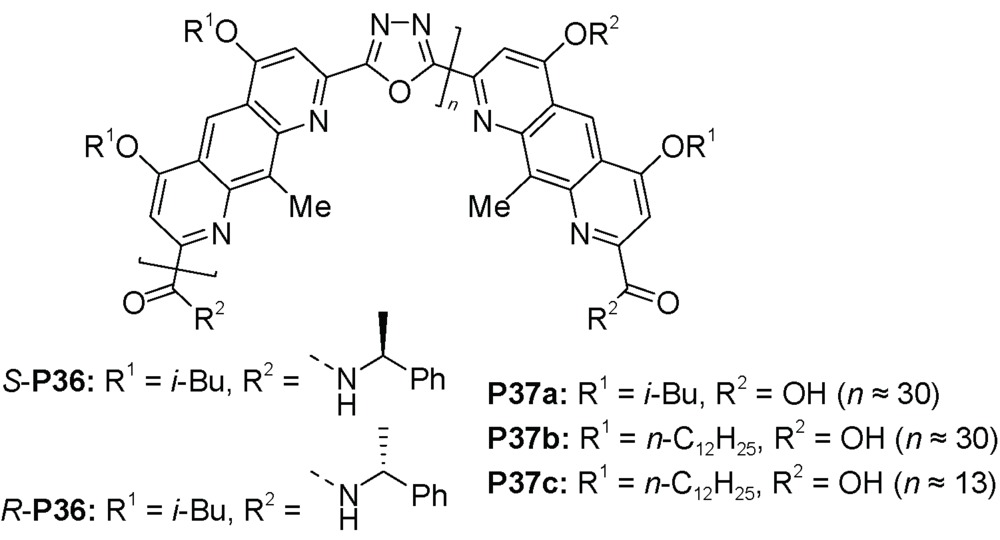
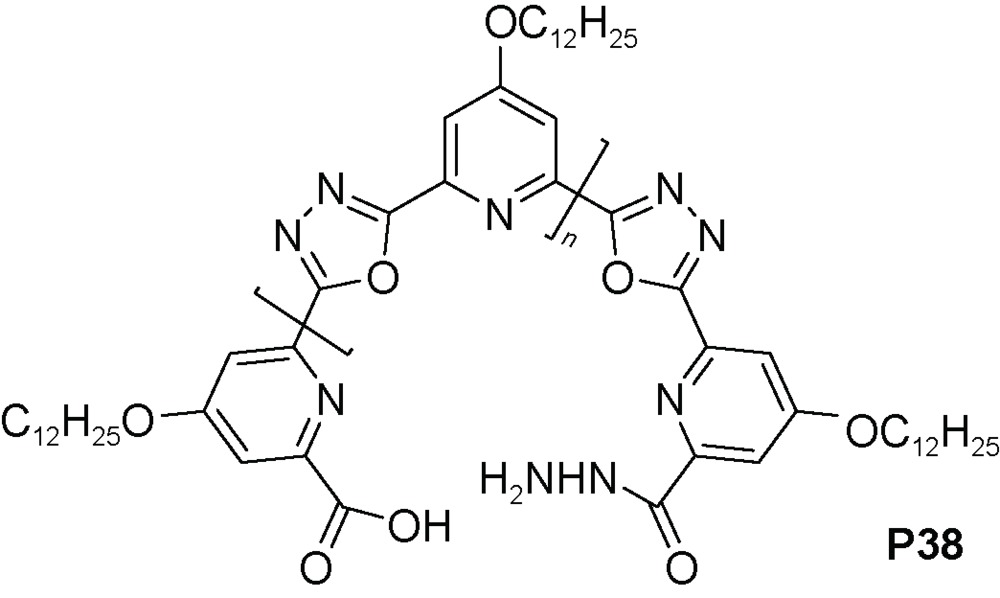
C) 氢键被破坏,聚合物P32a不能形成螺旋结构。3.2 三氮唑和口恶二唑构筑基元
3.3 间-苯乙炔和杂环类似物构筑基元
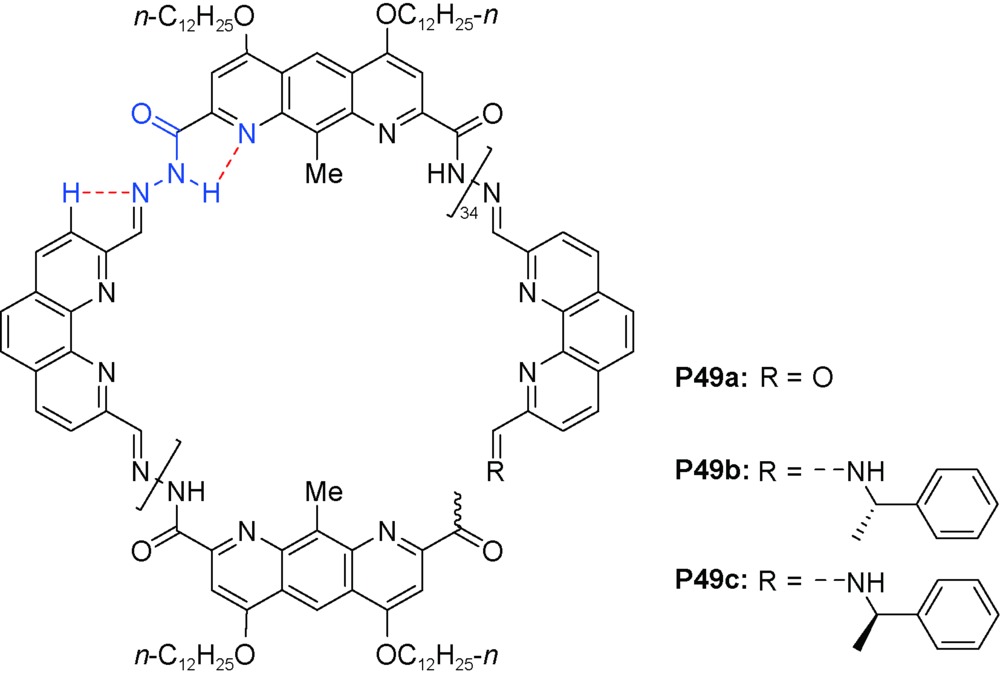
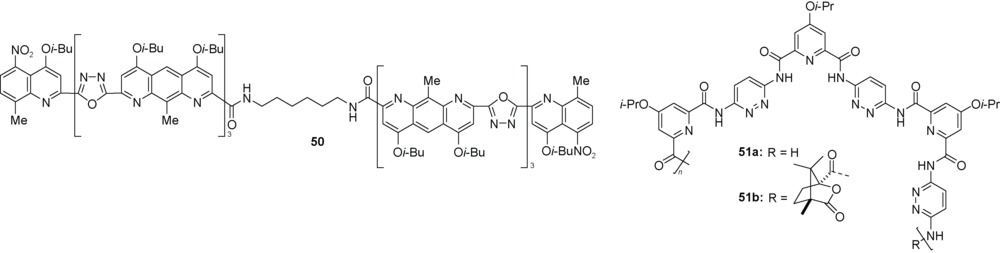
4 芳香超分子螺旋管
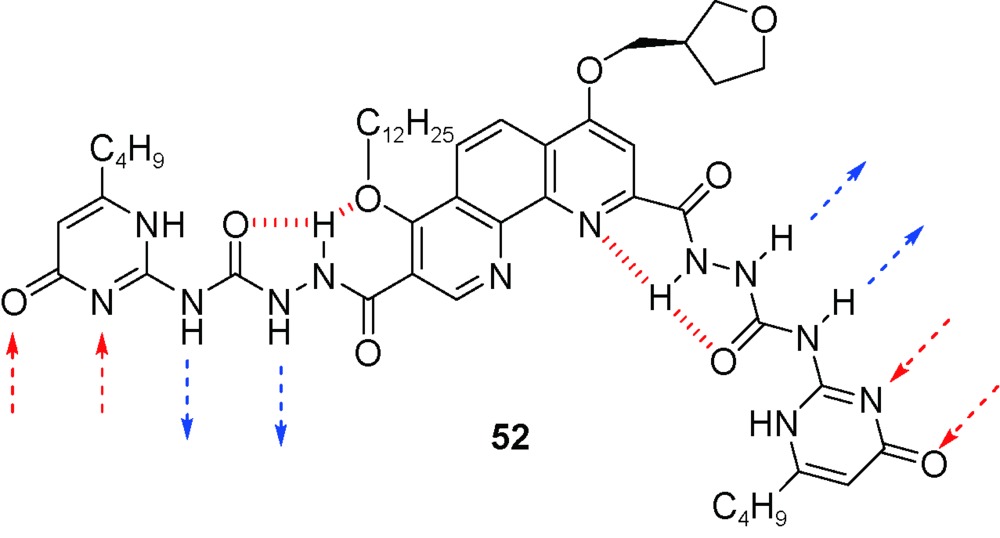
 O与另一个53a分子N-端苄氧羰基的苯基CH形成分子间(Bn)C—H…O(
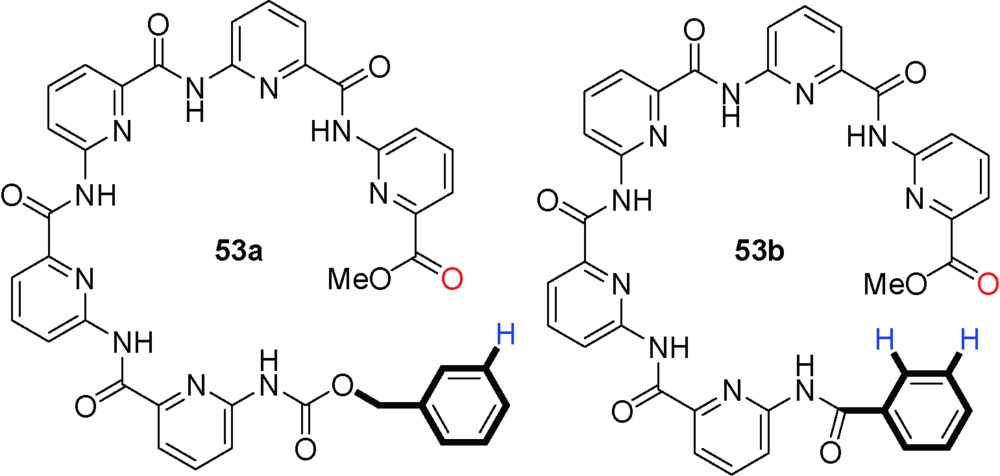
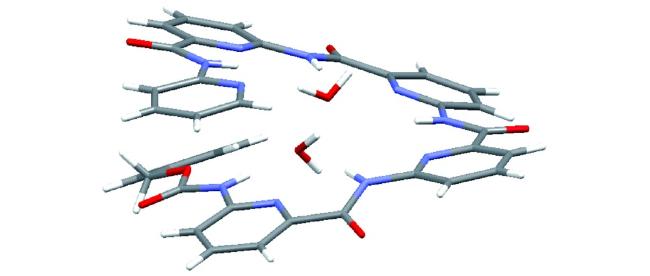
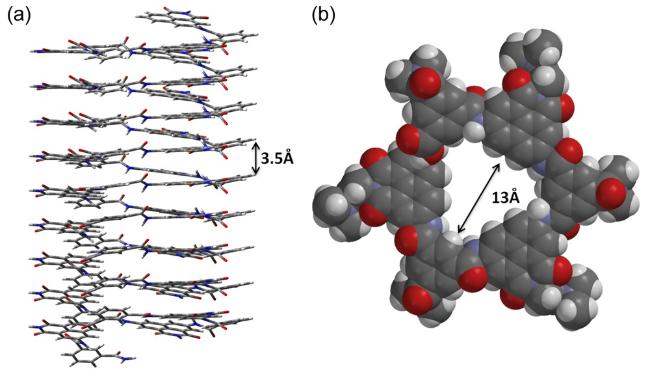
O与另一个53a分子N-端苄氧羰基的苯基CH形成分子间(Bn)C—H…O(  C)氢键作用,这种弱相互作用(晶体结构显示,氢键键长为2.44 Å)使53a分子之间发生“端到端”的粘结,所有寡聚体片段均有相同的手性,从而构建了超分子螺旋管。53a的超分子螺旋体空腔中形成了线性排列的甲醇分子(图7)。在含水溶剂中进行单晶培养,空腔中主要结合的仍是甲醇分子,仅有24%~40%的甲醇被置换为水分子。说明对小分子的包结有高度选择性。将N-端的苄氧羰基置换为苯酰基的53b,其通过分子间三中心(Ph)H2…O(
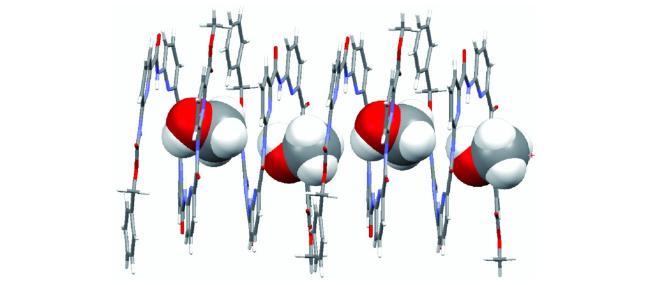
C)氢键作用,这种弱相互作用(晶体结构显示,氢键键长为2.44 Å)使53a分子之间发生“端到端”的粘结,所有寡聚体片段均有相同的手性,从而构建了超分子螺旋管。53a的超分子螺旋体空腔中形成了线性排列的甲醇分子(图7)。在含水溶剂中进行单晶培养,空腔中主要结合的仍是甲醇分子,仅有24%~40%的甲醇被置换为水分子。说明对小分子的包结有高度选择性。将N-端的苄氧羰基置换为苯酰基的53b,其通过分子间三中心(Ph)H2…O(  C) 氢键的粘结形成线性结构(晶体结构显示,氢键键长为2.75 Å和2.82 Å),其空腔内径仅为2.8 Å,只能容纳水分子而形成水线(图8)[99]。相应的四聚体或者六聚体都不能发生这样的粘结,“端到端”发生粘结的两个氢键给受体也需要在电子效应和位阻效应方面相匹配。
C) 氢键的粘结形成线性结构(晶体结构显示,氢键键长为2.75 Å和2.82 Å),其空腔内径仅为2.8 Å,只能容纳水分子而形成水线(图8)[99]。相应的四聚体或者六聚体都不能发生这样的粘结,“端到端”发生粘结的两个氢键给受体也需要在电子效应和位阻效应方面相匹配。