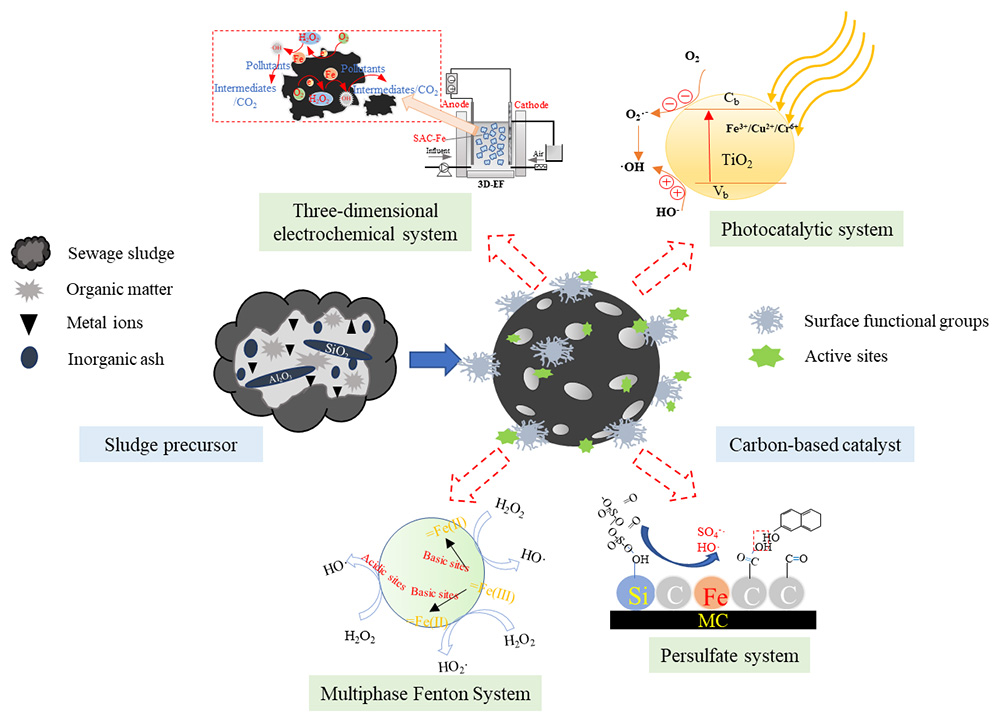
1 引言
2 污泥碳基前躯体的物理化学性质
2.1 化学组成
2.2 孔隙结构
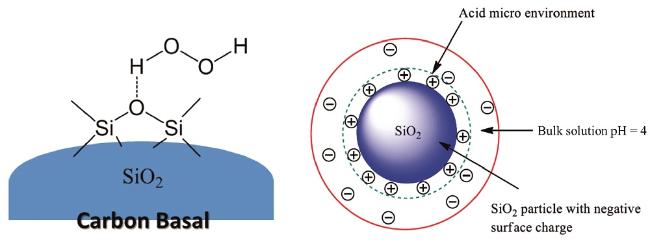
2.3 表面化学性质
表1 各种污泥碳基催化剂材料制备方法及其物理化学性质Table 1 A summary of the sewage sludge based carbon catalysts and their physiochemical properties |
| Catalyst | Preparation procedure | Method | BET surface area(m2/g) | Catalytic components | ref |
|---|---|---|---|---|---|
| FeSC | Dried sludge impregnated into 0.5 M FeSO4 solution and subsequently carbonized at 800 ℃ in the presence of N2 | Wetness impregnation | 14.3 | Magnetite, Quartz, Al2O3 | 19 |
| SC | Dried sludge carbonized at 800 ℃ in the presence of N2 | Direct pyrolysis | 57.6 | Carbon, ash | 14, 22 |
| SBC | Dried sludge impregnated into 3 M ZnCl2 solution and carbonized at 700 ℃ in the presence of N2 wash: 3 M HCl | Wetness chemical activation | 363 | Carbon | 23 |
| FMSAC | Carbonized sludge(by ZnCl2 pre-activation) was co-precipitated with Fe2+ and Fe3+ with NaOH addition | Chemical activation plus co-precipitation | 880~940 | Fe3O4, CaO, Quiz | 24 |
| R1 | Solid mixing of dried sludge with FeCl3(w/w=1∶1) and subsequently carbonized at 700 ℃ wash: 3 M HCl | Solid chemical activation | 517~836 | Fe species | 25 |
| szSAC | Dried sludge impregnated into the mixture of 3 M H2SO4 and ZnCl2 and then carbonized at 550 ℃ wash: 10% HCl | Wetness chemical activation | 179.9 | Surface-OH | 18 |
| szSAC/Mn | szSAC impregnated into KMnO4 solution and carbonized at 550 ℃ in the presence of N2 | Wetness impregnation | 3.7~11.7 | Mn(Ⅱ), Mn(Ⅲ), Mn(Ⅳ), surface-OH | 18 |
| DR-SA-A | Dried sludge was firstly carbonized with N2 and then activated with steam at 838 ℃, acid washed. | Steam activation | 497.4 | Dissolved organic matters and iron | 26 |
| nO x /SBAC FeO x /SBAC | Dried sludge was firstly activated with ZnCl2 and carbonized at 700 ℃ and had acid washed, then the carbonized products was impregnated into Mn/Fe solutions and re-carbonized at 600 ℃ | Chemical activation and wetness impregnation | 327~339 | Mn3O4, Fe3O4 | 15 |
| CFA/SC | Combined ZnCl2 activation and carbonization at 800 ℃ for the mixture of sewage sludge and fly ash(3 M HCl wash) | Chemical activation | 415 | Fe2O3, SiO2, Al2O3 | 27 |
| SS-Ti-700 | Combined hydrothermal reaction with TiOSO4 and carbonization at 700 ℃ | Hydrothermal reaction | 35.46 | TiO2, α-Fe2O3 | 28 |
| FAS-1-350 | Dried sludge impregnated into(NH4)2Fe(SO4)2 solutions, separated and calcined at 350 ℃ in the air | Wetness impregnation | 15.17 | α-Fe2O3, SiO2 crystallites | 29 |
| MC600 | Combined microwave digestion and KOH activation, then carbonized in the N2 | Chemical activation | 378 | O-containing groups, Fe3O4, α-Fe | 30 |
| SC-F-0.2 | Combined Fenton’s activation and carbonization at 600 ℃ | Radical activation | 46.3 | Fe3O4, α-Fe | 31 |
3 污泥碳基催化剂材料制备方法
3.1 直接热解法
3.2 负载法
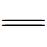 O、—COOH、—NH2等,这些官能团与变价金属结合形成更稳定的金属有机物骨架M—O—C,增强了催化剂稳定性,又可以通过电子传导增加金属表面的电子云密度,强化金属的催化活性[42]。根据被负载的对象不同,普通的负载法又可以分为碳化-负载-活化和负载-碳化的两段式和单段式工艺,常见的活性组分一般为FeCl3,Fe(NO)3,FeSO4,KMnO4和Mn(NO3)2等。
O、—COOH、—NH2等,这些官能团与变价金属结合形成更稳定的金属有机物骨架M—O—C,增强了催化剂稳定性,又可以通过电子传导增加金属表面的电子云密度,强化金属的催化活性[42]。根据被负载的对象不同,普通的负载法又可以分为碳化-负载-活化和负载-碳化的两段式和单段式工艺,常见的活性组分一般为FeCl3,Fe(NO)3,FeSO4,KMnO4和Mn(NO3)2等。3.3 共混法
3.4 水热碳化法
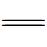 O键,极易与污泥中的活性金属如铁、钛、锌等结合,通过分子间缩聚形成新的带有催化活性中心的水热碳[47];同时,污泥中溶解的铁作为路易斯酸极易催化污泥水解并在水热碳基表面形成大量的含氧有机官能团,这些含氧官能团与金属活性物种具有极强的亲合力,能够抑制活性组分在载体表面发生团聚、降低活性组分的颗粒粒径、并强化活性组分在碳载体上的分布,阻止反应过程中活性物种在极端条件下向溶液相中的迁移[48],这种活性组分与碳基物料的化学吸附还可以促进物料在高温热解时的成孔作用,这将允许反应物在界面附近快速迁移至催化活性位点附近,从而提高催化剂的反应活性。
O键,极易与污泥中的活性金属如铁、钛、锌等结合,通过分子间缩聚形成新的带有催化活性中心的水热碳[47];同时,污泥中溶解的铁作为路易斯酸极易催化污泥水解并在水热碳基表面形成大量的含氧有机官能团,这些含氧官能团与金属活性物种具有极强的亲合力,能够抑制活性组分在载体表面发生团聚、降低活性组分的颗粒粒径、并强化活性组分在碳载体上的分布,阻止反应过程中活性物种在极端条件下向溶液相中的迁移[48],这种活性组分与碳基物料的化学吸附还可以促进物料在高温热解时的成孔作用,这将允许反应物在界面附近快速迁移至催化活性位点附近,从而提高催化剂的反应活性。4 污泥碳基催化剂材料的表面改性
5 污泥碳基催化剂在水环境催化领域的应用
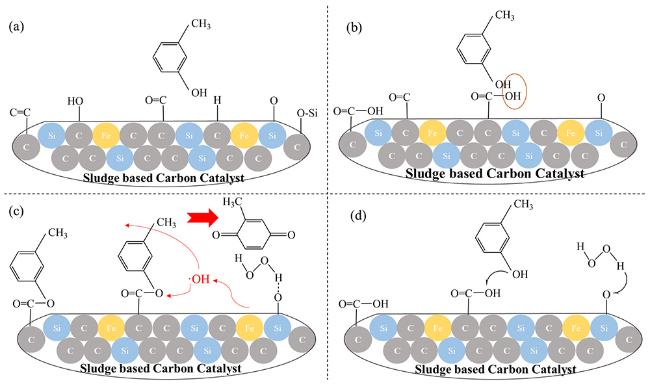
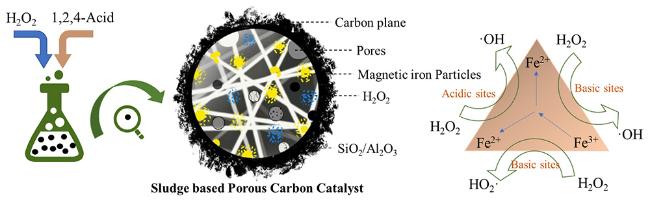
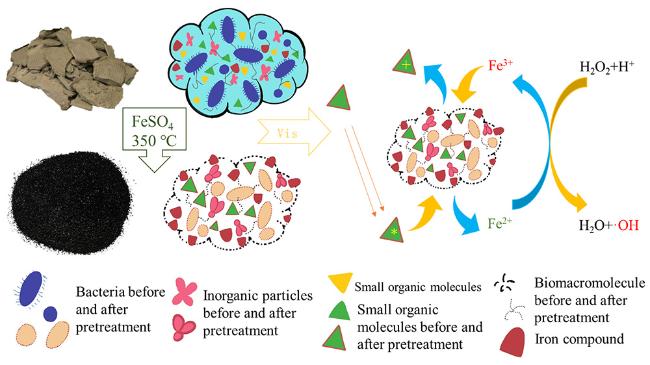
5.1 催化H2O2的多相类Fenton反应
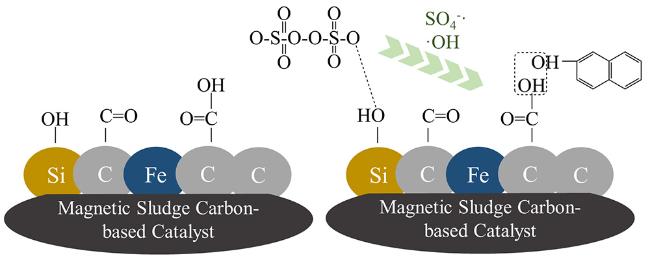
5.2 基于过硫酸盐(PS)和过一硫酸盐(PMS)的催化降解
 O官能团对PS的活化最为明显,利用甲醇和叔丁醇对自由基的淬灭试验证实,体系中同时存在·OH和S
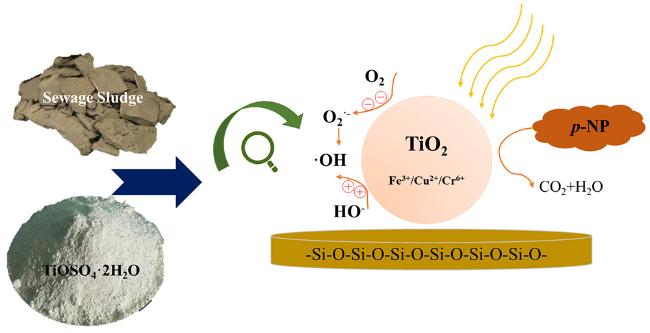
O官能团对PS的活化最为明显,利用甲醇和叔丁醇对自由基的淬灭试验证实,体系中同时存在·OH和S 5.3 复合光催化反应
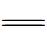 N—发色团断裂,并使染料褪色。
N—发色团断裂,并使染料褪色。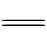 Fe(Ⅲ),加速
Fe(Ⅲ),加速 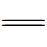 Fe(Ⅲ)催化H2O2产生羟基自由基。同时,Si-OH和Al-OH作为供电子基团,可以进一步被氧化断裂成羟基自由基。
Fe(Ⅲ)催化H2O2产生羟基自由基。同时,Si-OH和Al-OH作为供电子基团,可以进一步被氧化断裂成羟基自由基。5.4 非均相催化臭氧氧化
5.5 非均相催化湿式氧化
5.6 电化学催化氧化
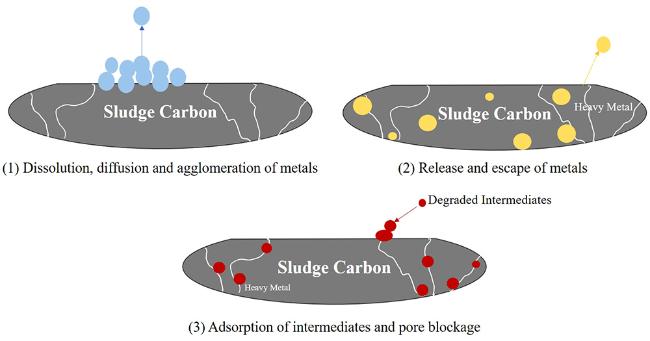
6 污泥碳基催化剂的稳定性
表2 几种污泥碳基材料在不同催化体系中的稳定性和可重复利用性比较Table 2 Comparison of stability and recyclability of several sludge carbonized materials in different catalytic systems |
| Leaching Species | Leaching Concentration | Reaction | Recyclability | ref |
|---|---|---|---|---|
| Fe | 0.6 g/L(2.5%of the total Fe load) | CWPO | 14.2% of the Fe load | 25 |
| Fe | not detectable | CWPO | 96% degradation efficiency obtained in third cycles of reaction | 92 |
| Fe | 0.037 mg/L(0.14% of total Fe loaded) Ca: 0.813 Cu: 0.029 Mg: 0.271 Zn: 0.027 | CWPO | 97% removal of AOII until at least 600 min | 19 |
| Fe | 1.9 mg/L for HNO3 treated SW 2.1 mg/L for H2SO4 treated SW 1.2mg/L for HCl treated SW 0.7 mg/L for SW | CWPO | 26.3% conversion of cresol for HCl-SW at 432 h 100% conversion of cresol for H2SO4-SW at 432 h 85.1% conversion of cresol for HNO3-SW at 432 h 40% to lower than 10% conversion rate for SW | 52 |
| Fe | 10.8 mg/L for HNO3 treated SW 11.7 mg/L for H2SO4 treated SW 0.8 mg/L for HCl treated SW 1.2 mg/L for SW | CWAO | Not mentioned | 7 |
| Fe | Fe: 18 mg/L Ni: 12 mg/L Zn: 4 mg/L Mn: 3 mg/L Cr: 3mg/L Mg: 2 mg/L | CWAO | For the fourth experiment, the differences after 4 h of reaction only amounted to 2.2% for phenol conversion and 9% for TOC conversion. | 26 |
| Fe | 27 mg/L(7% of the total Fe load) | CWAO | After 4 runs, the 2-CP conversion and the TOC removal were still very high. | 14 |
| Fe | 4.32 mg/L for SS-Fe-105 after 60 min 0.66 mg/L for SS-Fe-350 after 30min | Photo-Fenton | No obvious deactivation of the SS-Fe-350 catalyst in the six repetitive experiments was observed when compared with the first cycle. | 28 |
| Zn, Cu | 0.014 mg/L | PMS | The distributions of these heavy metals were unchanged though the MnO x /HCAS catalyst was reused up to 5 cycles. | 93 |
| Fe | pH 2.03:4.69 mg/L(2.14% of total iron) pH 3.01:3.06 mg/L | PS | three times for the oxidative degradation of AO7 | 65 |
| Fe | total Fe: 3.01 mg/L; Fe3+: 2.12mg/L | CWPO | It was observed that the mineralization rate decreased from 60.6 to 46.5% when the degradation rate of NOR decreased from 98.8 to 76.4%. | 94 |