Contents
1 引言
2 手性自组装多肽分子的设计
2.1 多肽分子序列的设计
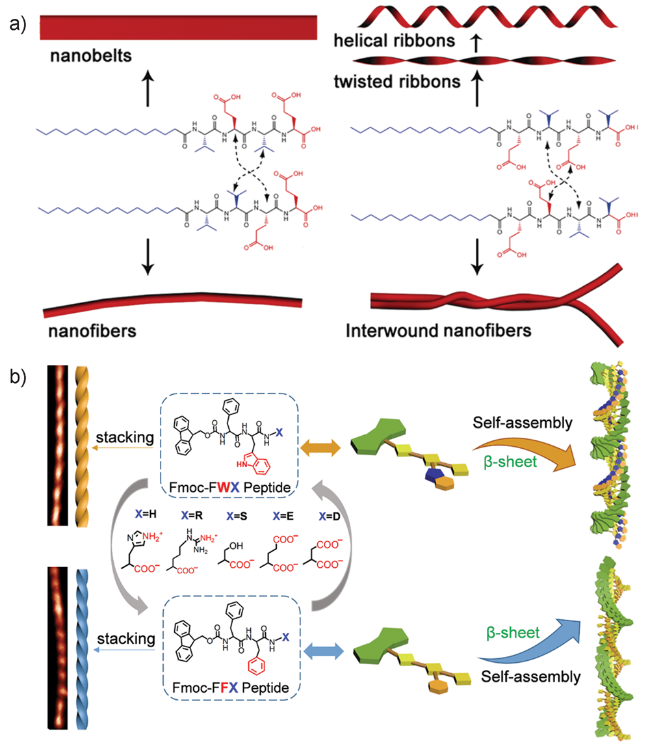
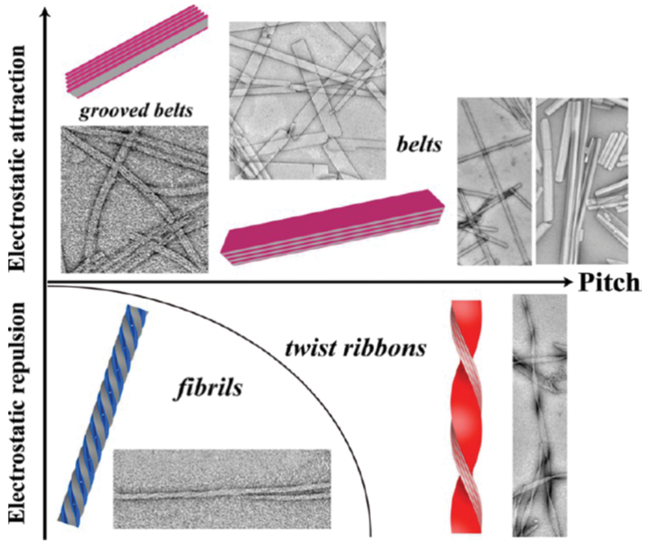
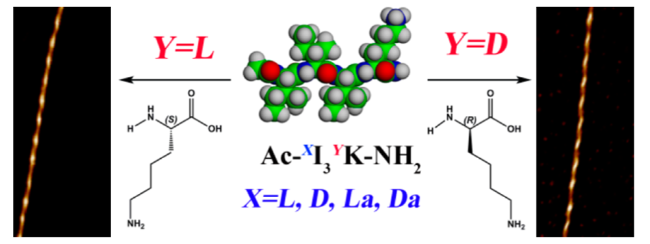
2.2 多肽单体组成氨基酸残基构型
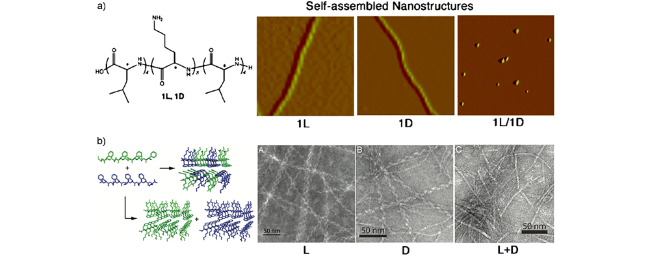
图4 多肽对映异构体单独组装及共组装示意图[51,52].(a)含有L-或D-氨基酸的三段型肽形成不同结构的自组装体;(b)对映体Ac-(FKFE)2-NH2肽共组装形成具有β-折叠结构的纳米带(1)或自组装形成对映手性的纳米螺旋(2)Fig. 4 Schematic illustration showing the self-assembly process of enantiomer polypeptides[51,52].(a) Atomic force microscopic images showing the triblock-type peptides composed of L- or D-amino acid self-assemble into different nanostructures;(b) Schematic and TEM images showing the enantiomeric Ac-(FKFE)2-NH2 peptides assemble into β-sheet nanoribbons(1) or self-sorted enantiomeric nanohelices(2) |
3 组装环境对多肽手性自组装行为的影响
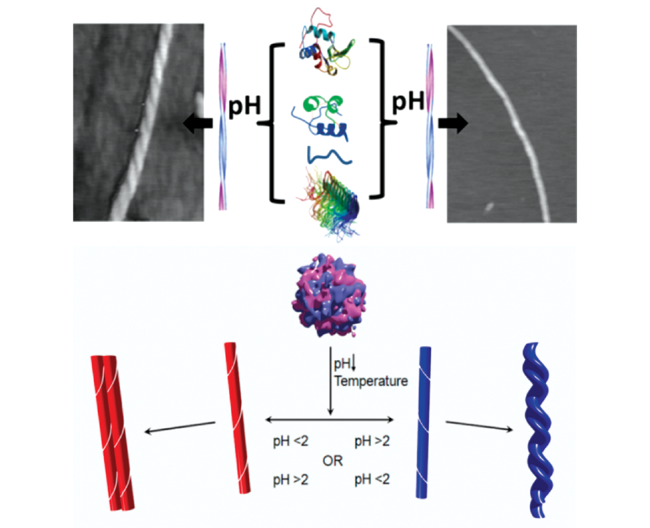
3.1 组装pH值
图6 不同pH下S233HisL12和S263His2L12手性组装体的透射电镜图(a~h)和组装示意图(i)[54]。由S263His2L12在10 mM TBS(pH=7.4,a和b)和10 mM CBS(pH=5.0,c和d;pH=3.0,e和f)中组装形成的纳米结构;由S233HisL12在10 mM CBS(pH=3.0,g和h)中组装形成的纳米结构Fig. 6 TEM images(a~h) and schematic illustration(i) of the chiral nanostructures self-assembled by S233HisL12 and S263His2L12 at different pH[54]. The molecular assemblies were prepared from S263His2L12 in 10 mM TBS(pH=7.4, a and b) and 10 mM CBS(pH=5.0, c and d; pH=3.0, e and f); The molecular assemblies were prepared from S233HisL12 in 10 mM CBS(pH=3.0, g and h) |
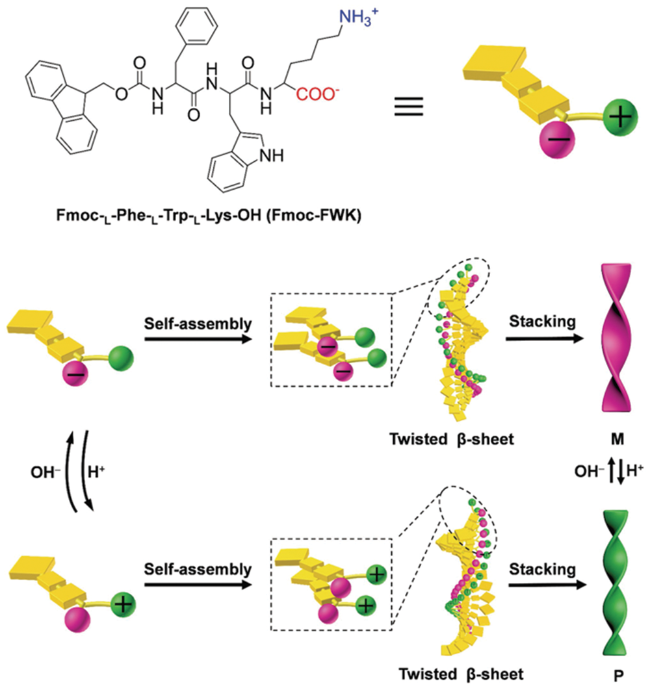
3.2 溶剂
3.3 添加剂:金属离子与对离子
图9 (a)谷氨酸衍生物4BLGA与不同金属离子形成凝胶宏观及微观形貌图[60];(b)由不同金属离子的L型对映体单体(LPF和LPPG)组装而成的纳米结构[61]Fig. 9 (a) Photo images of the 4BLGA gels with different metal ions and the SEM images of helical nanofibers formed by 4BLGA with different metal ions[60];(b) Nanostructures assembled from L-type enantiomeric monomers(LPF and LPPG) with different metal ions[61] |
3.4 光诱导多肽自组装
图11 (a)光响应Azo-GFGH分子结构式和肽基水解酶模拟物的催化性能[66];(b)阳离子苯丙氨酸二肽与偶氮苯衍生物超分子组装体的光响应特性示意图[67];(c) 酪氨酸-酪氨酸紫外光交联制备具有荧光特性的中空纳米囊和自支撑薄膜[68]Fig. 11 (a) Molecular structures of the designed Azo-GFGH and the photo-switchable assembly and the catalytic properties of the peptide-based hydrolase mimic[66];(b) Description of Photo-response Characteristics of Supramolecular Assembly of Cationic Phenylalanine Dipeptide and Azobenzene Derivative[67];(c) Synthesis of fluorescent hollow nanocapsule and free-standing thin lamella film by tyrosine-tyrosine UV crosslinking[68] |
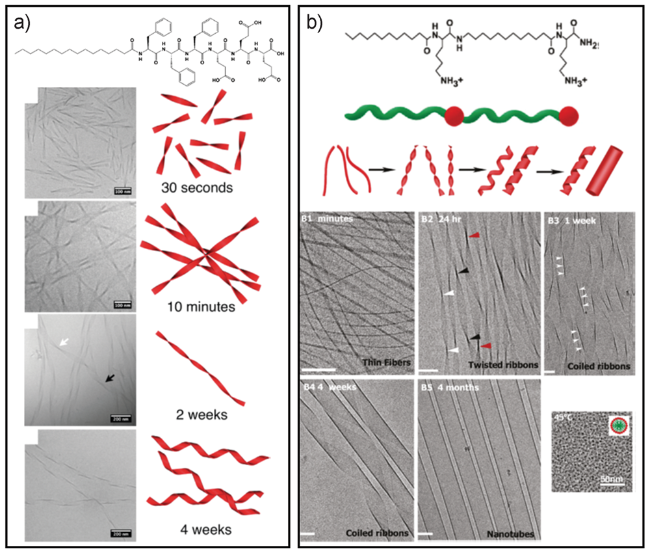
3.5 其他因素
图12 (a)一种两亲肽分子随时间变化形成手性组装体形貌的透射电镜图及示意图[73];(b)C12-β12手性自组装形貌随时间变化示意图[74]Fig. 12 (a) Cryo-TEM images and schematic illustration of the self-assembled helical assemblies of the peptide amphiphile at different time[73];(b) Cryo-TEM images and schematic illustration of pathway to nanotubes by chiral C12-β12 self-assembly[74] |
4 肽类手性自组装材料应用
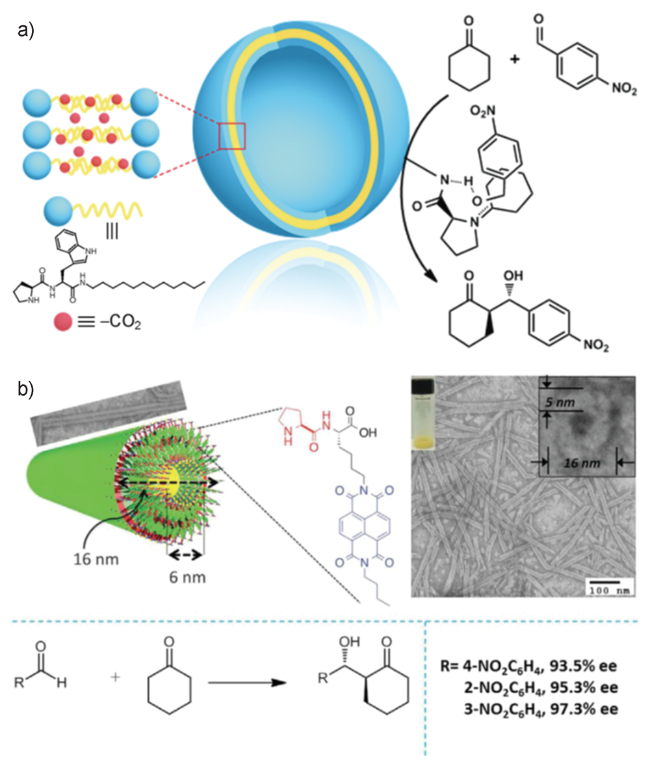
4.1 手性催化
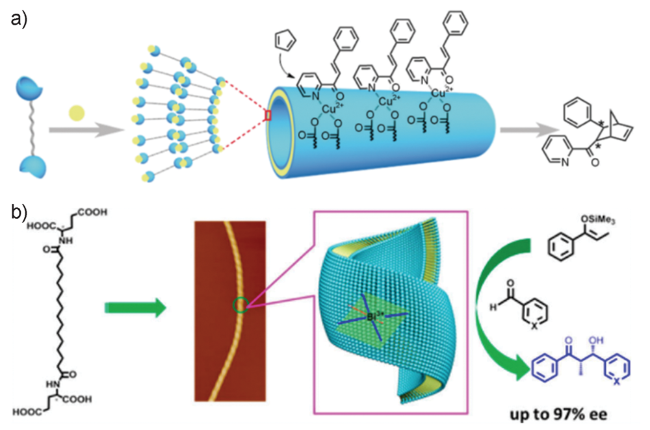
图14 (a)Cu2+-肽共组装体催化剂催化不对称Diels-Alder反应和(b)Bi3+-肽共组装体催化剂催化不对称aldol反应示意图[35,77]Fig. 14 The schematic illustration of the assembly mechanism of(a) Cu(Ⅱ)-HN catalysis and its asymmetric catalysis for Diels-Alder reaction and(b) Bi(Ⅲ)-HN catalysis and its asymmetric catalysis for Mukaiyama Aldol reaction[35,77] |
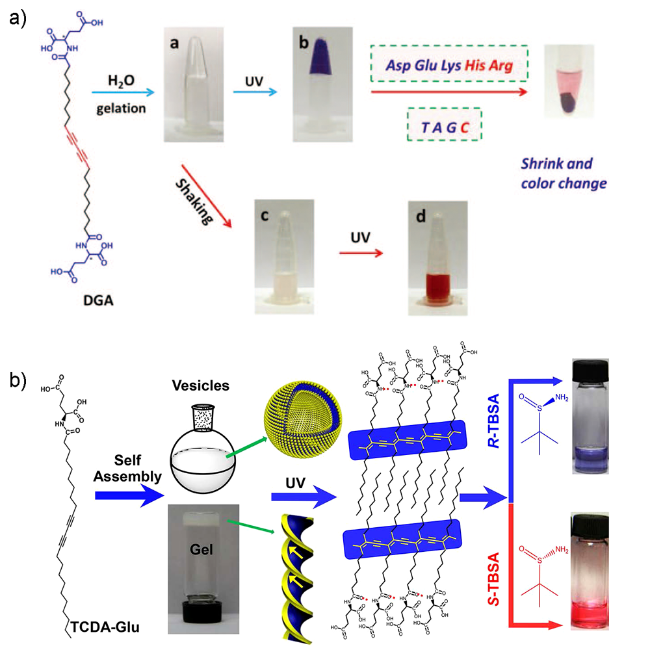
4.2 手性检测
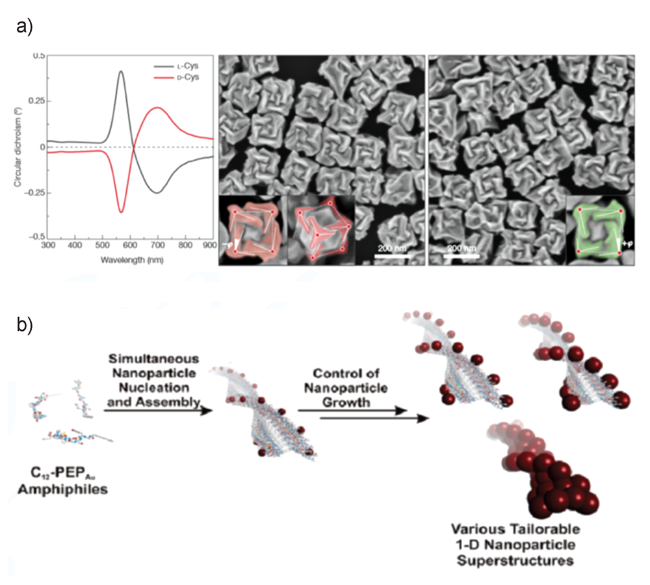
4.3 手性模板
4.4 手性光学
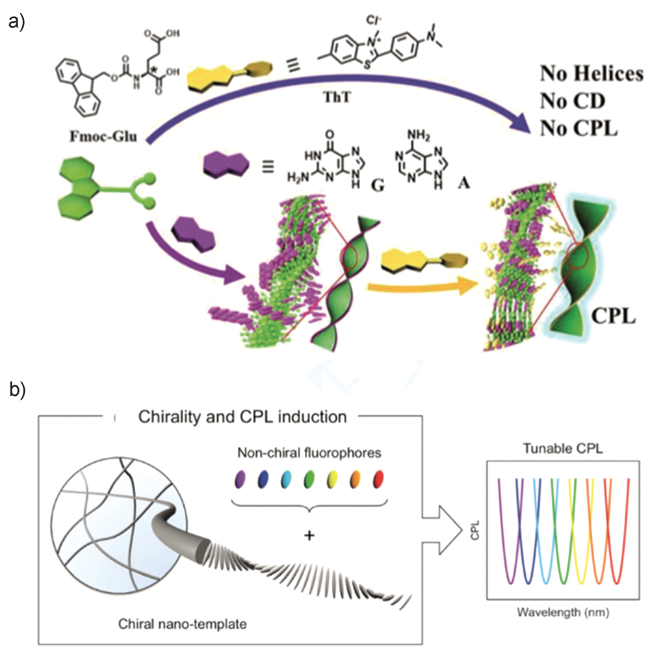
图19 (a)Fmoc-Glu与嘌呤核苷自组装成螺旋结构及手性从Fmoc-Glu到ThT的手性转移示意图[90]; (b)谷氨酸衍生物作为纳米模板手性诱导荧光染料产生CPL示意图[91]Fig. 19 (a) Self-assembly of Fmoc-Glu and purine nucleosides into helical structures and chiral transfer from Fmoc-Glu to ThT[90];(b) Chiral Induction of Fluorescent Dyes by Glutamic Acid Derivatives as Nanotemplates[91] |