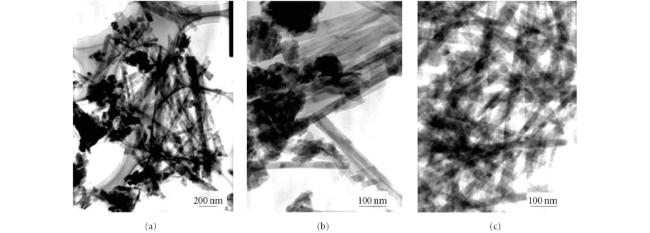
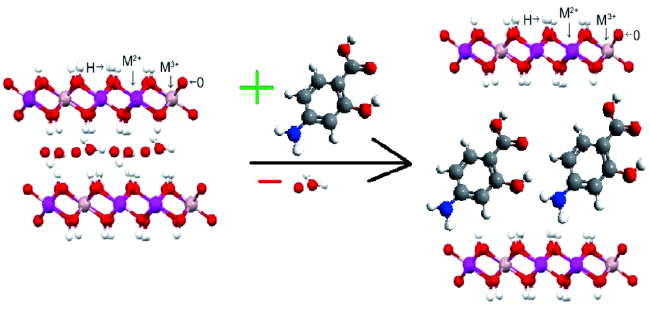
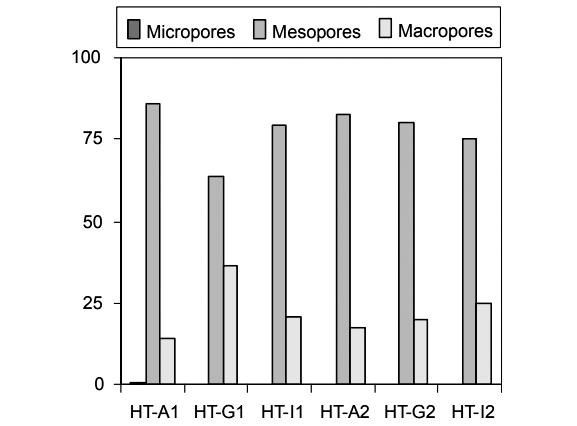
Contents
1 Introduction
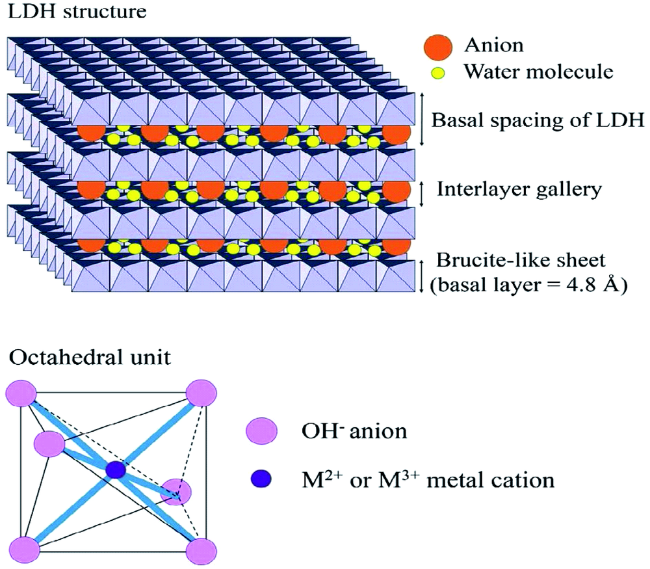
2 Structure
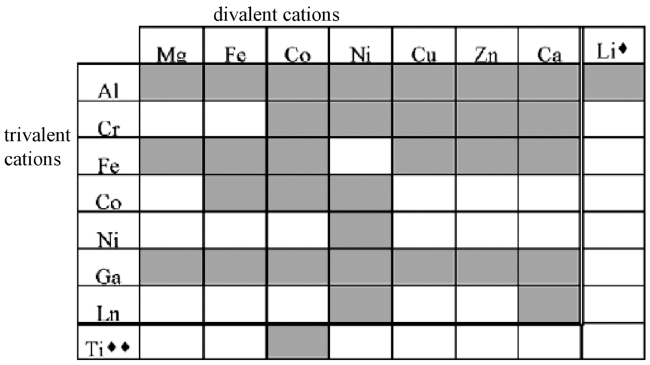
2.1 Composition
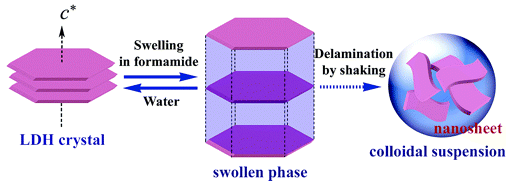
2.2 Delamination process
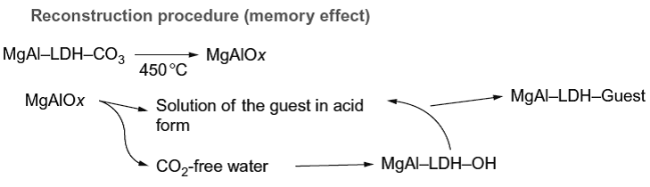
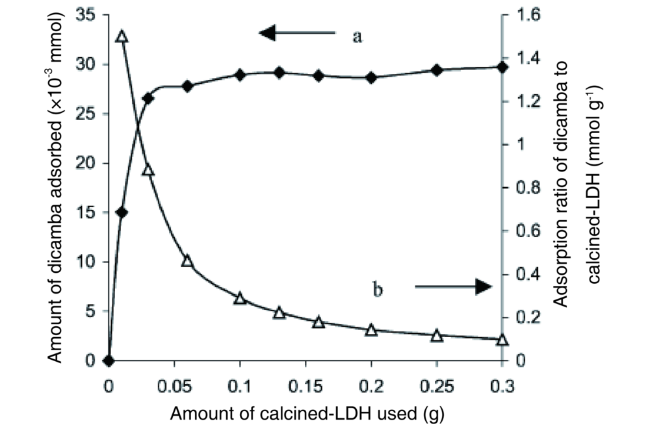
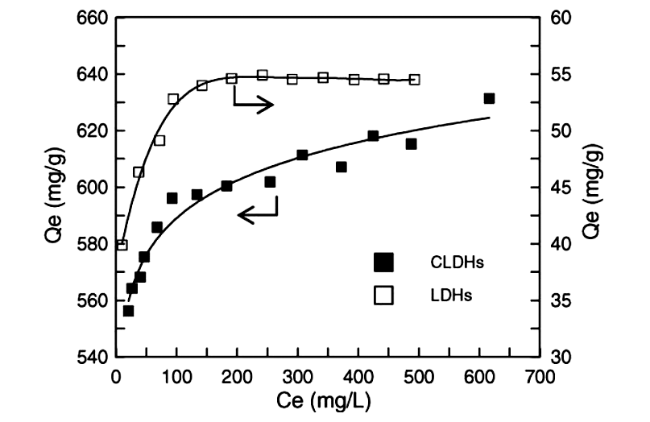
2.3 Calcination/memory effect/reconstruction
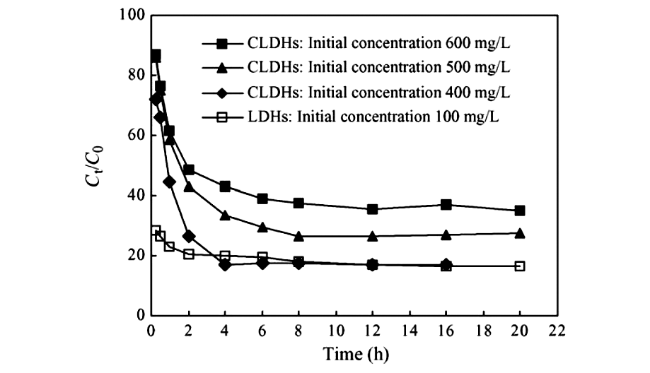
Fig.6 Effect of contact time on the uptake of Brilliant Blue R(BBR) by layered double hydroxides(LDHs) and calcined LDHs(CLDHs) at different initial concentrations[43] |
2.4 Pillared LDHs
3 Properties
3.1 Physical properties
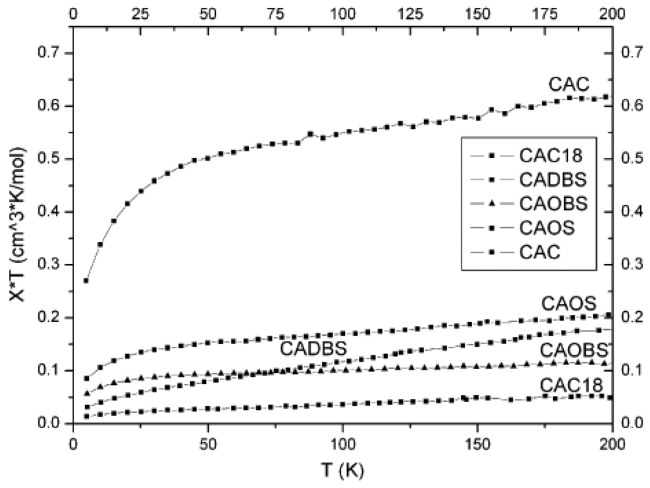
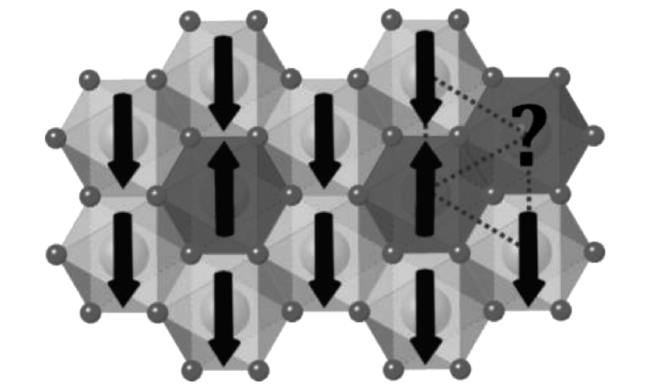
3.2 Magnetic properties
4 Preparatory methods
| Mineral | Structural Formula | Intercalated ion | Cell Parameters(Å) | Space Group |
|---|---|---|---|---|
| Fougerite | Fe42+Fe23+(OH)12[CO3]·3H2O | OH-, Cl-, CO32- are possible | a=3.17~3.18 c=22.7~22.9 | R$\bar{3}$m |
| Meixnerite | [Mg6Al2(OH)16][(OH-)2.4H2O] | OH- | a=3.046 c=22.93 | R$\bar{3}$m |
| Zincowoodwardite | Zn1-xAlx(OH)2[SO4]x/2·nH2O x<0.5, n<3x/2 | SO42- | a=3.063 c=8.91 and a=3.065 c=25.45 | P$\bar{3}$m and R$\bar{3}$m |
| Hydrotalcite | [Mg6Al2(OH)16][(CO32-)·4H2O] | CO32- | a=6.13 c=46.15 | R$\bar{3}$m |
| Pyroaurite | [Mg6Fe3+(OH)16][(CO32-)·4H2O] | CO32- | a=6.19 c=46.54 | R$\bar{3}$m |
| Mössbauerite | Fe63+O4(OH)8[CO3]·3H2O | CO32- | a=3.07 c=22.25 | R$\bar{3}$m |
| Charmarite | Mn4Al2(OH)12[CO3]·3H2O | CO32- | a=10.98 c=15.10 | P6322 |
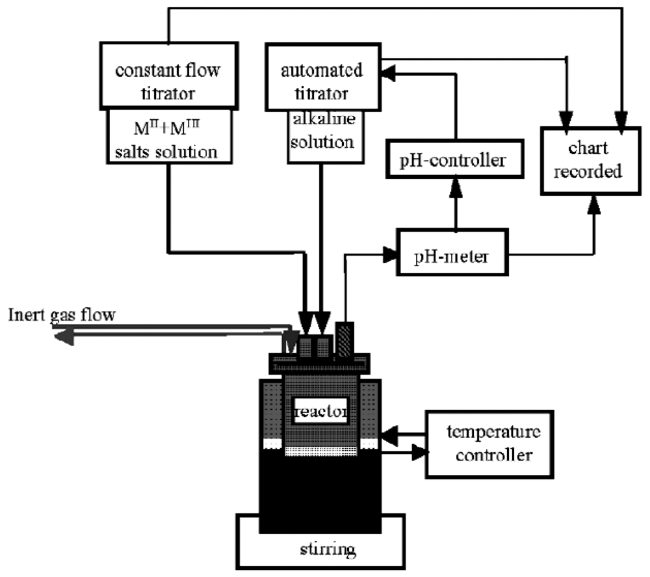
4.1 Co-precipitation method
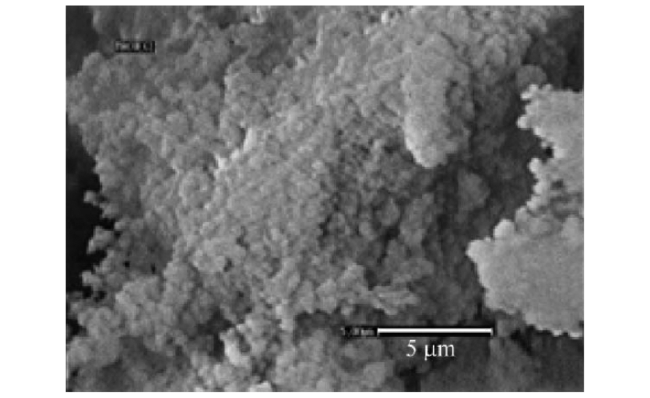
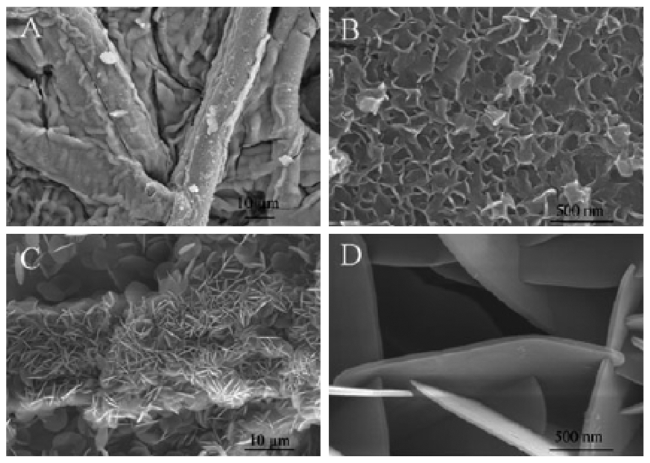
Fig.13 The SEM micrographs of the(a) ZnAl-4,(b) CZnAl-4-300 C and(c) CZnAl-4-500 C sample. The arrows in the left figures indicate where the enlargements(in the right figures) were taken. The marked spots(in the right figures) indicate the part taken for the EDX measurements[69] |
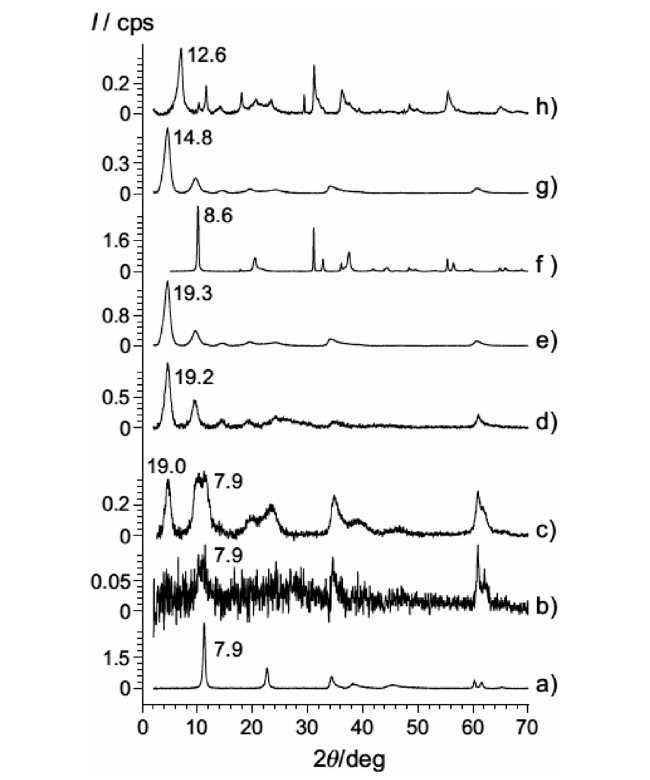
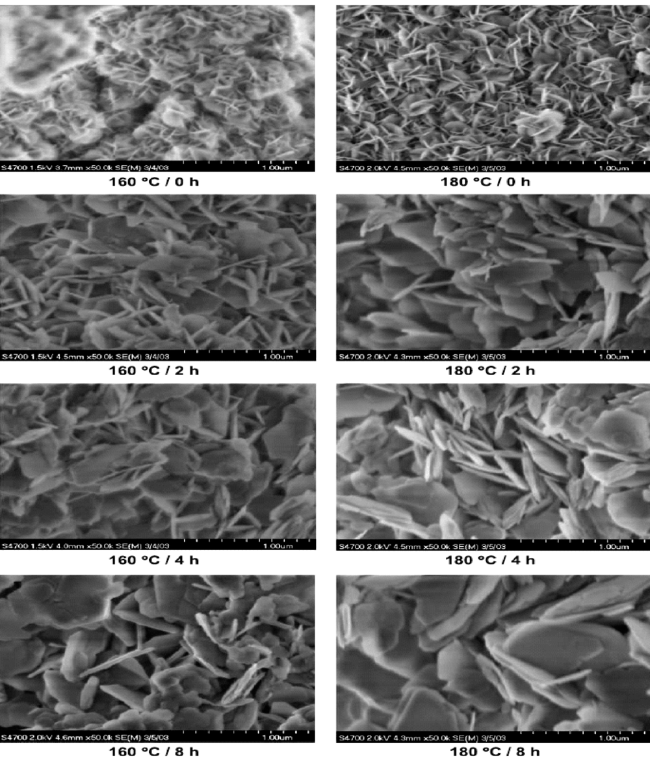
4.2 Hydrothermal approach
4.3 Anion exchange method
4.4 Urea hydrolysis
4.5 Sol-gel method
5 Application
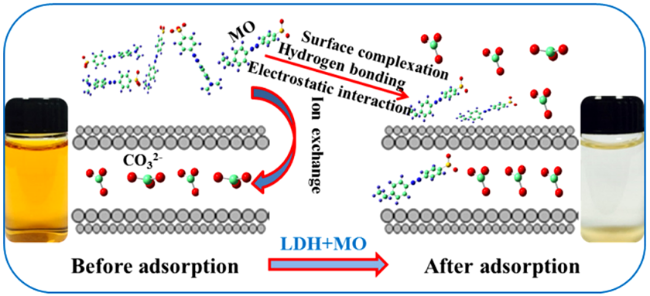
5.1 Adsorbents and anion exchangers
Table 2 A brief literature of layered double hydroxide with different metal combinations |
| Metals-anion | Preparation methods | Charac. techniques | ref |
|---|---|---|---|
| Ca-Al-NO3 | Co-precipitation | PXRD, FTIR | 96 |
| Co-Fe-OH Co-Fe-OH/CO32- (pyroaurite group) | Topochemical synthesis Co-precipitation method | XRD, SEM, TEM, AFM XRD, FTIR, Mossbauer spectroscopy, DTA | 97 98 |
| Co-La-CH3COO- | hydrogen peroxide catalyzed hydrolysis reaction | FTIR, PXRD, TGA, PL spectroscopy | 99 |
| Cu-Al- CO32- | Hydrothermal approach | FTIR, PXRD, TGA | 100 |
| Zn-Cr-Cl-/CO32-/NO3- | Co-precipitation | UV-VIS analysis, PXRD, FTIR | 101, 102 |
| Zn-Ti-NO3 Zn-Al-CO3 Zn-Al-CO32-/NO3- | Co-precipitation Co-precipitation Co-precipitation at low supersaturation | TEM, SEM, PXRD, XPS SEM, TGA, PXRD, TEM SEM, XRD, EDX, IR, TG/DTG | 103 56 104 |
| Ni-Fe-Cl | Topochemical synthesis | SEM, TEM, PXRD, XPS, FTIR | 105 |
| Mn-Al-CO32-/NO3-/SO42-/Cl- | Co-precipitation | PXRD, FTIR, DTA/TG, SEM | 106 |
| NiTi-CO32- | Co-precipitation at high supersaturation | PXRD, SEM, ICP-AES, FTIR | 68 |
| Mg-Al-CO32- | Hydrothermal approach Urea hydrolysis | SEM, XRD, TGA, FTIR XRD, FTIR, BET | 71, 72, 84, 83 |
| Zn-Al LDH films | Urea hydrolysis Sol-gel route | XRD, SEM, TEM XRD, SEM | 81 89 |
| Ni-Al/Mg-Al-CO32- | Sol-Gel method | XRD, SEM, TEM, HRTEM | 90 |
| Zn-Al LDH | Sol-gel method | XRD, TG-DSC | 91 |
| Mg-Al/Ga/In | Sol-gel method | FTIR, XRD, DRIFT | 94 |
| Ni-Co-Al/Mg-Ni-Al | Sol-gel method | XRD, TEM, TGA-DTA | 95 |