 PDF(4402 KB)
PDF(4402 KB)


高分子类型MONOLITH材料的制备技术及其作为亲和色谱固定相用于分离生物大分子的应用
许颖, 高婷婷, 王启晓, 屈阳, 刘宏飞, 辛渊蓉
化学进展 ›› 2018, Vol. 30 ›› Issue (8) : 1112-1120.
 PDF(4402 KB)
PDF(4402 KB)
 PDF(4402 KB)
PDF(4402 KB)
高分子类型MONOLITH材料的制备技术及其作为亲和色谱固定相用于分离生物大分子的应用
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Preparation Technologies of the Polymer-Based MONOLITH Material and Its Application as Stationary Phase of Affinity Chromatography for the Separation of Biological Macromolecules
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}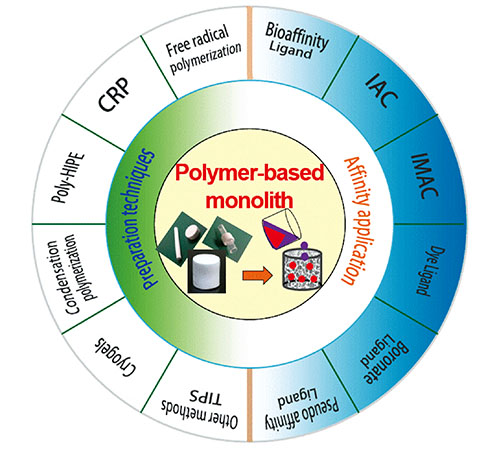
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |